Introduction
Hypophthalmichthys molitrix, also named silver carp, is a popular planktivorous fish species in freshwater aquaculture for its faster growth rate and lower feeding cost than other fish species.1 It is also introduced into ponds, reservoirs, and lakes worldwide to control the phytoplankton biomass, prevent blooms, and promote nutrient regeneration. In China, silver carp is one of the Four Major Chinese carp, with a total production of 3.81 million tons in 2020, which was the second-highest production fish among all fish species. However, intensive aquaculture practices and the deterioration of water quality often give rise to bacterial diseases in fish, causing economic loss to the aquaculture industry.2 The commonly reported bacterial pathogens in silver carp are Plesiomonas shigelloide,3 Aeromonas sobria,4 and Acinetobacter spp., which have become the limiting factors for the silver carp aquaculture industry.
The genus Aeromonas is composed of various species, mainly pathogens of various fish species, and is widespread in the aquatic environment worldwide.5 Aeromonas veronii, an important opportunistic pathogen, could cause diseases in various kinds of fish, including largemouth bass Micropterus salmoides,2 crucian carp Carassius auratus,6 sheatfish Silurus glanis,7 Nile tilapia Oreochromis niloticus8,9 and channel catfish Ictalurus punctatus.10 The clinical signs caused by A. veronii include hemorrhagic ulcers, abdominal ascites, abdominal distension, redness, and swelling of the anus (He et al., 2019). In 2011, A. veronii was reported in silver carp for the first time.11 At the same time, there were limited reports on the infection of A. veronii in silver carp from then on, especially regarding its pathogenicity.
In May 2018, a disease was an outbreak in wild silver carp in Yantian Reservoir, Guangdong Province, China, which resulted in mass mortality of female silver carp with eggs. We preliminarily considered that the death of the fish was caused by bacterial disease and isolated an A. veronii strain from infected female silver carp. The present work aimed to identify the pathogenic bacteria, study its pathogenicity and conduct histopathological and immune-related enzyme activity analyses.
Materials and Methods
Isolation and identification of the bacteria strain
Moribund broodfish of silver carp weighing 0.5-1.5 kg (n=22), with a body length of 31-35 cm, were sampled from Yantian Reservoir, Guangdong province, China, where broken out fish disease. The temperature, dissolved oxygen, and pH in water were 26.5°C, 9.5 mg/L, and 8.4, respectively. The fish were transported immediately to the laboratory to isolate pathogenic bacteria. The infected fish were anesthetized with 100 mg/L of tricaine methanesulfonate (MS-222), then their skins were disinfected with 75% ethyl alcohol. Next, the blood samples were collected from the caudal vessel, and the ascites in the ovary were collected post-dissection. The obtained blood and ascites samples were spread on the plates containing beef extract-peptone solid medium (10 g/L peptone, 3 g/L beef extract, 5 g/L NaCl, 16 g/L agar, pH 7) to isolate pathogenic bacteria.12 The plates were cultured at 28°C for 24 h. The dominant bacteria were purified using streak cultivation on the beef extract-peptone solid medium three times. The single colony named WSQ-1 was selected and inoculated in beef extract-peptone broth at 28℃ for 18 h. The bacterium solution was preserved at -80℃ after adding sterile glycerol at a final concentration of 40% (v/v).
Morphological and biochemical characterization
Morphological observation of the WSQ-1 isolate strain was carried out using Gram staining. Biochemical identification and characterization of the isolated strain WSQ-1 were carried out using 21 biochemical and physiological test reactions, the standard strain Aeromonas veronii GDMCC 1.893 was purchased from Guangdong Microbial Culture Collection Center. The identification of WSQ-1 was according to the method of the Identification Manual of Common Bacterial Systems.13 The details of these 21 biochemical tests are shown in Table 1.
Molecular characterization
Genomic DNA of WSQ-1 was extracted using an UNIQ-10 column DNA extraction kit (Sangon, China) following the manufacturer’s instructions. A pair of universal primers 27F: 5’-AGAGTTTGATCCTGGCTCAG-3’ and 1492R: 5’-GGTTACCTTGTTACGACTT-3’15 were used for amplification of the 16S rRNA gene, gyrB3F: 5’-TCCGGCGGTCTGCACGGCGT-3’ and gyrB14R: 5’-TTGTCCGGGTTGTACTCGTC-3’ were used for amplification of the gyrB gene.16 The PCR amplification conditions were initial denaturation at 94℃ for 4 min, followed by 30 cycles of denaturation at 98℃ for 30 s, annealing at 54℃ for 45 s, and extension at 68℃ for 45 s, as well as a final extension at 68℃ for 7 min. The amplified products were analyzed with 1% agarose gel electrophoresis, and the sequence was cloned into the pMD-18T vector (TakaRa, Japan) and sequenced by BGI Genomics (Shenzhen City, China). A blast search for the sequence was carried out via the NCBI website. The phylogenetic tree was established using the Neighbor-Joining method in the MEGA 6.0 software package.
Experimental infection
Healthy juvenile silver carp weighing 30-50 g were purchased from a commercial fish farm at Huadu, Guangzhou City, Guangdong province. 180 healthy adult silver carp weighing 1.5-4 kg were obtained from Zhenkou Reservoir, Jiangxi province, China. Before the experiment, the fish were treated with copper sulfate at a concentration of 2 mg/L for about 20 min to eliminate all pathogens on fish gills and surfaces. Five individuals of the fish were randomly selected for bacterial and parasitic examination to ensure that the fish had no pathogen, then were temporarily reared for a week in 9 of 2 cubic meters tanks with aerated and dechlorinated freshwater in the Aquatic Animal Technology Laboratory of Jinan University, each tank contained 20 fish and 1.8 cubic meter water. The healthy adult and juvenile silver carp were fed daily with powder feed at 1% of fish weight. The water temperature was kept at 23.0 ± 0.3°C with an air conditioner, the dissolved oxygen was higher than 6.0 mg/L, and the pH of the water was 8.1.
A total of 240 healthy juvenile silver carps (30-50 g) were randomly divided into four groups: (a) Injected infection group: each silver carp was injected with 100 µL WSQ-1 suspension by intraperitoneal injection at a concentration of 3×106 CFU/mL 17; (b) Bathed infection group: the fish were bathed with WSQ-1 at a concentration of 3×107 CFU/mL; (c) Injected control group: each fish was injected with 100 µL 0.65% saline water; (d) Bathed control group: the fish were treated with 0.65% saline water. Each group was carried out in triplicate.
Female fish with eggs (n=90) were divided into an infected group (n=45), and a control group (n=45), and male fish (n=90) were also divided into an infected group (n=45) and a control group (n=45). Each group contained three tanks with 15 fish in each tank. The fish have injected with 100 µL WSQ-1 suspension at a concentration of 3×107 CFU/mL per kilogram in infected groups and injected with 100 µL 0.65% saline water per kilogram in the control group.
The bacteria were sub-cultured in 10 ml of beef extract-peptone medium, and the suspension was incubated at 28°C for 24 h. After incubation, the culture was centrifuged for 5 min at 5000 rpm. The supernatant was removed, and the bacteria were washed twice with normal saline (NS) solution and then resuspended in 10 ml of NS. Next, 1 ml of cell suspension dilution was made up to 10−7 in NS, and the diluted suspension was spread on the medium and cultured at 28°C for 24 h to determine the number of cells/. All fish were raised at 23℃ for 14 days to observe and record pathological symptoms. The fish were inspected daily for clinical signs and mortality. The blood and ascites from infected fish were collected to re-isolate the pathogenic bacterium.
Histopathological examinations
The spleen, liver, and gonads of moribund fish from the artificially and naturally infected silver carp were sampled. The collected samples were then fixed in Bouin’s fixative, dehydrated in ethanol, embedded in paraffin wax blocks, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E) for histopathological observation under a light microscope.18
Serum enzymes activity
Blood samples from the healthy, artificially infected, and naturally infected fish were obtained from the caudal vein after the fish were anesthetized with 100 mg/L MS-222. Then the collected blood was centrifugated at 1469 g for 10 min at 4°C. The serums were obtained and stored at −20°C before use. The serum activities of acid phosphates (ACP), alkaline phosphatase (ALP), and catalase (CAT) were measured using Kits (Nanjing Jiancheng Bioengineering Institute, China) following the instructions.
Statistical analysis
The data were analyzed with Excel 2016 and SPSS Statistics 24 (International Business Machines Corporation). The comparison of the difference between experimental groups and control groups was analyzed by chi-square test and one way-ANOVA. A significant difference was considered at p < 0.05. Sequence analysis was performed using MEGA 6.0 software.
Results
Clinical symptoms of naturally infected fish
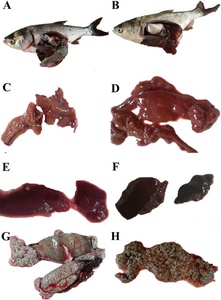
Twenty-eight silver carp were collected in May 2018, of which 22 were sexually mature females with eggs, and all had diseased symptoms, while the male individuals showed no clinical symptoms. Clinical symptoms of the naturally diseased fish showed abdominal distension and anal congestion. After dissection, a substantial amount of ascitic fluid filled the abdominal cavity, and the fish’s liver and spleen were swollen and darkened. The ovary membrane was broken, then the eggs scattered and changed from slight to full transparency (Figure 1).
Biochemical characterization
WSQ-1 was isolated from naturally diseased fish with colony characteristics of grey-white, smooth surface, the translucent, slightly convex, and regular margin on the agar plates (Figure 2A). The strain belonged to a Gram-negative bacterium with red color and blunt ends without spores (Figure 2B). The physiological and biochemical characteristics of WSQ-1 were the same as those of the standard strain. The esculin hydrolysis and ornithine decarboxylase results were negative, and the arginine dihydrolase and L-Arabitol results were positive, which indicated that WSQ-1 was consistent with Aeromonas veronii biovar veronii. Thus, the pathogenic bacterium WSQ-1 was identified as Aeromonas veronii biovar veronii (Table 1).
Molecular identification of the isolate
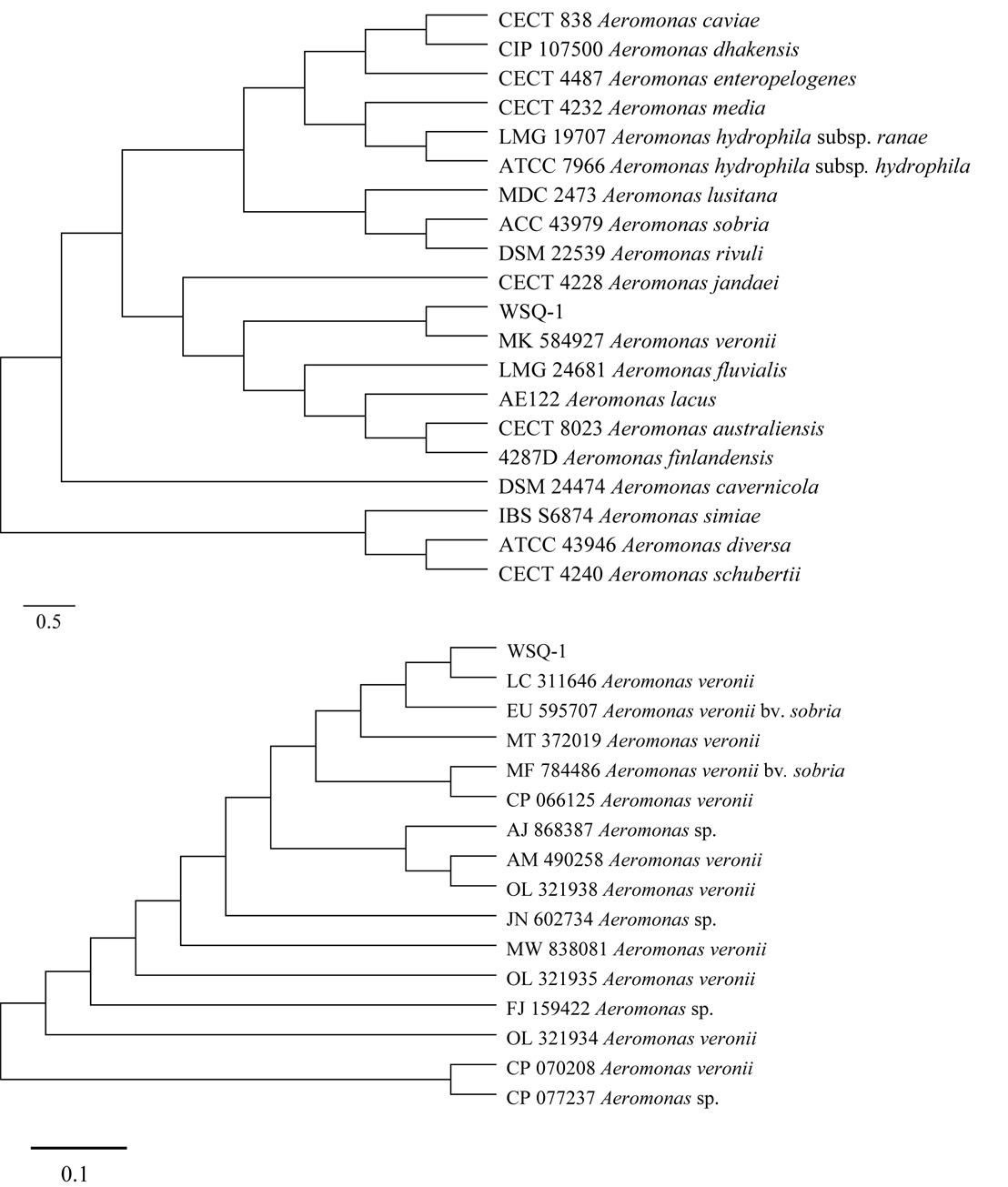
The taxonomic position of the isolated bacterium was based on sequence analysis of the 16S rDNA and gyrB. The phylogenetic tree indicated that WSQ-1 belonged to the Aeromonas and clustered into an independent branch with A. veronii (MK584927.1) (Figure 3A) and A. veronii (LC311646.1) (Figure 3B). The NCBI blast results indicated that WSQ-1 shared 99.5% and 98.41% identity with the reference strain A. veronii.
Experimental infection
The present results indicated that A. veronii caused 100% death of juvenile silver carp challenged with intraperitoneal injection and the pathogenic bacterium suspension bath. In addition, A. veronii resulted in 100% mortality of sexually matured female silver carp with eggs post intraperitoneal injection, but it caused no mortality of sexually matured male individuals (Table 2). The clinical signs of infected female fish or juvenile fish showed an enlarged abdomen with ascites accumulation in the abdominal cavity and hyperemia of the pectoral fins and abdomen. The male fish showed no clinical signs. The pathogen was re-isolated from the ascites and blood of the infected fish and identified as A. veronii. The bacteria were re-isolated from the female rather than the male fish.
Immune-related enzyme activity
The ACP, ALP, and CAT serum enzyme activities showed no significant difference between healthy mature females with eggs and healthy mature females without eggs (p > 0.05). Still, they decreased significantly in the diseased mature females with eggs (p < 0.05) (Table 3). In the artificial infection of silver carp, the serum ACP, ALP, and CAT levels dropped significantly on day 6 (Table 4).
Histopathological findings
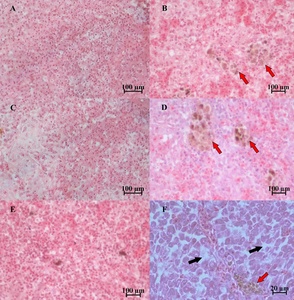
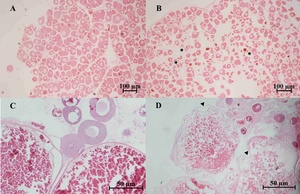
Severely damaged tissues could be detected in both of naturally and artificially infected female silver carp. The livers and spleen showed hemosiderin deposition and vacuolar degeneration (Figures 4 and 5). The ovary histopathological section revealed egg cells’ disordered arrangement, wavy membrane deformation, and even broken egg cells (Figure 6).
Discussion
Aeromonas species, opportunistic pathogens, are widespread in aquatic environments and can cause acute or chronic infection in aquatic animals.10 Among them, Aeromonas hydrophila is the pathogenic bacterium mainly studied, resulting in significant economic loss in aquaculture. While other isolates, such as A. veronii, A. sobria, A. caviae, and A. punctate, are also potential pathogens of freshwater fish in aquaculture.8 Aeromonas spp. infection usually causes similar clinical symptoms of diseased fish, such as hemorrhage, ascites, redness, and swelling of the anus. For example, A. veronii results in ulcerative syndrome, fin rot, and hemorrhagic septicemia in freshwater fish19; A. hydrophila causes hemorrhagic ascites, enlargement of the swim bladder, as well as intestinal and renal congestion of the moribund fish20; A. sobria infection leads to the clinical symptoms of redness and swelling of the anus, severe hemorrhage of the intestine in fish.4 Thus, it is difficult to distinguish the practically pathogenic Aeromonas species according to the clinical symptoms of diseased fish. The pathogenic bacterium isolated from diseased silver carp was identified as Aeromonas veronii biovar veronii with morphological and biochemical characteristics and 16S rDNA and gyrB gene DNA sequence analyses. The isolated strain was identified as *A. veronii**,* which had been reported in silver carp. However, its pathogenicity in the fish was still lacking.6,11 Therefore, the challenge experiments were performed to study the potential pathogenic ability of A. veronii in silver carp.
The healthy juvenile fish were challenged with A. veronii by intraperitoneal injection and bath with the strain suspension. The results indicated that silver carp showed 100% mortality in the infection group. The infected juvenile fish’s clinical symptoms were similar to those of the naturally infected fish, such as abdominal distension and anal congestion, and a substantial amount of ascitic fluid filled the abdominal cavity. A. veronii could be re-isolated from the infected host, establishing Koch’s postulates. These results demonstrated that the isolated strain was a pathogen.
During the investigation of A. veronii in Yantian Reservoir, all diseased and dead silver carp were sexually mature female fish with eggs. All sexually mature male fish, however, showed no A. veronii infection. This phenomenon was demonstrated using the challenge experiments. The results displayed that A. veronii resulted in 100% mortality of matured female silver carp and no death of the male fish. These results indicated that sexually mature female silver carp with eggs were much more susceptible to A. veronii than sexually mature male silver carp.
In mammals, the sex hormones of females during pregnancy cause the female to be vulnerable to pathogens, and the clinical and experimental evidence showed that estrogens affect the immunity of female individuals.21 A similar phenomenon was also reported in rainbow trout.22 Previous studies demonstrated an increment in plasma estrogen levels during the pre-spawning period of females.23 Elevating sex steroid levels made fish more vulnerable to pathogens during the spawning season, subsequently increasing fish mortality.24 In the present study, there was no sex difference in the mortality of juvenile fish. Only sexually mature female silver carp with eggs were infected by A. veronii, indicating that the sex steroid might cause the female fish to be more vulnerable to A. veronii. Further study should investigate the exact mechanism of the present phenomenon.
Furthermore, this study is the first report on the histological changes in silver carp infected by A. veronii. Aeromonas infections could result in various histological changes, such as cell necrosis, cell punctate vacuolation, cell swelling, necrosis, intravascular congestion, hepatocellular steatosis, and hemorrhage.25,26 The pathological changes of infected silver carp were characterized by septicemia and irreversible lesions to the spleen, livers, and ovary. Bacterial cells with high density caused tissue vacuolation (Gaafar et al., 2021). Hemosiderin accumulation might result from blood cell destruction, iron release from hemoglobin, and increasing hemosiderin.27 Cell membrane deformation and cells (eggs) broken in the ovary were also observed in other organs of fish infected by Aeromonas spp.8,26,28,29
The levels of various active enzymes in blood closely relate to fish’s health status and anti-infection immunity.30 ACP and AKP are the marker enzymes of macrophages, which are involved in the transfer and metabolism of phosphate groups. The level of ACP and AKP reflects the activation degree of macrophages. The reactive oxygen species (ROS) play an essential role in the anti-infection of invading pathogens, but the excessive production of ROS leads to oxidative stress damage to the body. CAT can protect the cells from the grievous effects of oxidative stress to avoid the adverse effects of ROS.31 A previous study indicated that AKP and CAT could be used as biochemical indexes to evaluate the resistance of tilapia to Streptococcus agalactiae disease. The lower expression levels of ALP and CAT indicated lower resistance after infection with Streptococcus agalactiae.32 Moreover, the increased CAT activity in the livers and ACP and AKP activities in the plasma enhanced the antioxidant capability, innate immunity, and disease resistance of Megalobrama amblycephala.6 The present study showed that the ALP, ACP, and CAT activities of H. molitrix kept a normal fluctuation range in healthy female fish but were decreased significantly in diseased matured female individuals with eggs. This suggested that the resistance was reduced significantly in the fish infected by the pathogenic bacterium. This was an essential factor to result in the death of diseased fish.
In conclusion, the present study demonstrated that the isolated pathogen WSQ-1 was identified as A. veronii biovar veronii, which was highly pathogenic to silver carp and caused the mass death of sexually mature female fish with eggs. The findings in this study will provide new insights into the diagnosis and the sexual susceptibility caused by A. veronii and help understand the pathogenesis of Aeromonas spp. in fish.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [32173013]; the Shellfish and Large Algae Industry Innovation Team Project of Guangdong Province [2022KJ146]; the Key-Area Research and Development Program of Guangdong Province [2019B020217001].
Conflict of Interest
The authors have no competing interests to declare.
Ethical Statement
The usage of fish was approved by the Jinan University Laboratory Animal Ethics Committee [20200511-11]. The authors followed the Guide for the Care and Use of Laboratory Animals and the National Standards of Laboratory Animals of the People’s Republic of China.