Introduction
Mud crab (Scylla paramamosain) is the most cultured species in the southeast coast of China. The advantage of S. paramamosain is rapid growth, delicious meat, and high nutritional value.1 However, as mud crab farming has increased, mud crab sickness has become a more serious concern (Cheng et al., 2021). Mud crab is not only under pressure from environmental factors such as temperature and heavy metals but also from viral diseases such as Scylla Serrata reovirus, Mud Crab Dicistrovirus-1, and bacterial pathogens such as Vibrio harveyi. Disease prevention for mud crabs is now an urgent issue. The use of antibiotics and chemicals not only damages the water column and leads to changes in plankton abundance, but may also have toxic effects on aquatic organisms and soil.2 Chinese herbal medicines have been increasingly researched and used to control disease because of their low residue and they are less susceptible to the development of drug resistance.3
Many Chinese herbal medicines promote the growth of aquatic animals, due to their antibacterial and germicidal effects, and also strengthen immunity and resistance to disease.4 Astragalus caudiculosus extract was found to improve the growth, antioxidant status, and immune response of rainbow trout.5 Additionally, a dietary Chinese herbal medicine mixture improved the growth performance, enteric trypsin activity, body protein content, non-specific immunity, and antioxidant capacity of European eels.6
Guava leaves are the dried leaves and leafy shoots of the guava tree (Psidium guajava L.), and contain a variety of chemical constituents such as flavonoids, quercetin, and terpenoids.7 Modern pharmacological research has demonstrated that guava leaves have antioxidant, antiviral, and antibacterial activities.8 Previous research demonstrated that guava-leaf extract can improve the immunity of Labeo rohita.9 Moreover, guava also greatly reduced the mortality rate of Oreochromis mossambicus and increased its resistance to Aeromonas hydrophila.10 Guava-leaf extract were also shown to provide considerable growth-promoting and immune-boosting effects in shrimp.11
Therefore, guava-leaf extract is a promising feed additive in aquaculture. Aqueous extracts of guava leaves are used as feed supplements to effectively increase the immunity of mud crabs. To improve the pharmacological studies on Chinese herbal medicine in the mud crab. To give a theoretical foundation for the manufacture of herbal medicines and feed additives. In this study, mud crabs were fed diets containing different concentrations of guava leaf aqueous extract to assess the effects on growth, digestion and antioxidant enzyme activity, and non-specific immunity.
Materials and Methods
Ethical statement
All experiments were approved by the Animal Care and Use Committee of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (No. SCSFRI96–253) and were performed according to the regulations and guidelines established by the committee.
Preparation of experimental feed
Crushed guava leaves (12 g) were decocted twice and the liquid was mixed, and filtered to remove residue. The filtrate was boiled over a gentle flame to 1.2 L. An aqueous extract of 10 g·L–1 of guava leaves was obtained. The guava-leaf extract of 0 mg·kg–1, 80 mg·kg–1, 160 mg·kg–1, 320 mg·kg–1, and 640 mg·kg–1 was added to the basal feed (Surgreen, Qingdao, China), stirred and mixed well. The diets were air-dried at 38°C in a drying cabinet and stored at 4°C.
Experimental animals and culture management
Mud crabs (15 ± 2.00 g) were purchased from Jiangmen, Guangdong Province, China. The water salinity was 10‰, temperature was 24 ± 2°C, and dissolved oxygen content was 6.50 ± 0.20 mg·L−1. Mud crabs were provided basic food twice daily until 24 hours prior to the start of the trial.
Five groups, including 0 mg·kg–1(control), 80 mg·kg–1, 160 mg·kg–1, 320 mg·kg–1, and 640 mg·kg–1 guava-leaf extract groups, each containing four parallel groups and 10 crabs each, were used in the experiment. To provide shelter and cover for the mud crabs, mock plants (seven forks of 32 leaves; height, 33 cm; spread diameter, 20 cm) were placed at random intervals in each box (0.20 m × 0.35 m × 0.35 m). All culture boxes had constant aeration, natural lighting, and darkness. Each day, feeding was provided at 8:00 and 17:00 at a rate of 8% to 10% of the crab mass. At 18:00, the water was changed at a rate of 30%.
Growth performance
At the beginning and the end of the 30-day feeding trial, all crabs were weighed and growth performance parameters were calculated using the formulas below. Before weighing, the crabs were fasted for 24 h.
Weight gain (WG, %) = (WT− W0)/W0 × 100;
Specific growth rate (SGR, %/day) = (ln WT− ln W0)/d × 100%;
Survival rate (SR, %) = (N0−NT)/N0 × 100%;
Where W0 and WT are the initial body weight (g) and final body weight (g) of crabs, respectively, and N0 and NT are the final numbers of crabs and an initial number of crabs, respectively.
Sample
After reared for 30 days and weighed, six mud crabs were randomly selected from each group, for a total of 30 mud crabs in five groups. The extracted mud crabs were put into ice for 1-3 minutes for low temperature anesthesia, then the mud crab body surface was disinfected with 75% alcohol, dissected and sampled. One sample of gastric tissue was taken from each mud crab, stored at -80°C and digestive enzyme activity was determined. One sample of hepatopancreas tissue was taken from each mud crab and stored at -80°C for determination of antioxidant enzyme and immune enzyme activities. Two parallel crab hepatopancreas tissues were combined into one sample, with three biological replicates per group, then hepatopancreas tissues were stored in RNA Sample Preservation Solution (TIANGEN, Beijing, China) at 4°C overnight and -80°C for long-term storage.
Digestive enzyme activity assay
Accurately weighed gastric tissue (0.1 g) was added to phosphate buffered saline (PBS) buffer and homogenized with a sample freezer grinder to obtain 10% gastric tissue homogenate, which was centrifuged at 3000 g for 15 min. The supernatant was collected for further analysis. Amylase (AMS) activity was measured using the method of Bernfeld.12 Pepsin activity was determined using casein as a substrate and reacting with Folin’s reagent (Anson M, 1933).13 Lipase (LPS) activity was determined using McKellar and Cholette.14 The reagents were used in commercial assay kits (JianCheng, Nanjing, China). All reagents are provided in kits.
Antioxidant enzyme activity assay
Accurately weighed hepatopancreatic tissue (0.1 g) was added to PBS buffer and homogenized with a sample freezer grinder to obtain 10% hepatopancreatic tissue homogenate, which was centrifuged at 8000 g for 15 min. The supernatant was collected for further analysis. Total antioxidant capacity (T-AOC) was measured by reducing Fe3+ to Fe2+, which forms a solid complex with pheophorbides. Superoxide dismutase (SOD) activity was measured by its ability to inhibit the superoxide anion produced by the xanthine and xanthine oxidase reaction system. One unit of activity was defined as the amount of enzyme required to produce a semi-inhibition of color formation at 450 nm. Malondialdehyde (MDA) levels were determined using the method described by Buege and Aust.15 Glutathione activity was determined colorimetrically and quantitatively by reacting with dithiodinitrobenzoic acid to produce a yellow compound. The reagents were used in commercial assay kits (JianCheng, Nanjing, China), all reagents are provided in kits.
Immunoenzymes
Accurately weighed hepatopancreatic tissue (0.1 g) was added to PBS buffer and homogenized with a sample freezer grinder to obtain 10% hepatopancreatic tissue homogenate, which was centrifuged at 5000 g for 15 min. The supernatant was collected for further analysis. Both alkaline phosphatase (AKP) and Acid phosphate (ACP) decompose sodium benzodiphosphate to produce free phenol and phosphate. To determine the level of enzyme activity, the phenol is oxidized in alkaline solution containing 4-amino antipyrine by potassium ferricyanide to produce a red quinone derivative. Lysozyme (LZM) activity was determined according to the method of Demers and Bayne. Briefly, hepatopancreas samples were mixed with Micrococcus lysodiekticus suspension in phosphate citrate buffer (pH 5.8), and the turbidity was read at 450 nm to calculate the lysozyme activity (μg /mL.). Glutathione (GSH) activity was measured using the colorimetric method. The reagents were used in commercial assay kits (JianCheng, Nanjing, China), all reagents are provided in kits.
Real-time PCR
The expression of GPx3, JNK, SOD, CAT, GST, and P53 genes in the hepatopancreas of crabs was measured. Total RNA was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer’s protocol. cDNA synthesis was performed using a PrimeScript RT reagent Kit with gDNA Eraser (Takara, Dalian, China). Real-time PCR was conducted using SYBR Premix Ex Taq II (Takara, Dalian, China) on a qTOWER384G Real-Time PCR Thermal Cycler (Analytik Jena, Germany). The initial denaturation was performed at 95 °C for 10 min, and elongation was performed at 95 °C for 5 s, and at 60°C for 20 s for 40 cycles. Each test was repeated three times. The RNA purity was assessed using a NanoDrop spectrophotometer (Thermo Scientific, USA), and the RNA integrity was checked by running the samples on a 1.2% agarose gel. The primer sequences used for real-time PCR are shown in Table 1. The data were analyzed using the 2-ΔΔCT method with 18s rRNA as the internal control.
Statistical analyses
Data were assessed by analysis of variance (ANOVA) with the quadratic model in SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Data were tested for normality using the Shapiro-Wilk test and for homogeneity of variances using Tukey tests prior to being subjected to ANOVA. Differences were considered significant at P < 0.05. The results are presented as the mean ± SE.
Results
Growth performance
The growth performance of the different experimental crab groups is presented in Table 2. Among all crab groups, the most favorable results were obtained for the 320 mg·kg–1 guava-leaf extract group. The WT in the 320 mg·kg–1 guava-leaf extract group was substantially greater than that in the control group (P < 0.05). The 320 mg·kg–1 guava-leaf extract group also had the highest SR (P < 0.05). WG and SGR showed an increasing trend followed by a decreasing trend, with the 160 mg·kg–1 and 320 mg·kg–1 guava-leaf extract groups being significantly higher than that of the control group (P < 0.05) (Table 2).
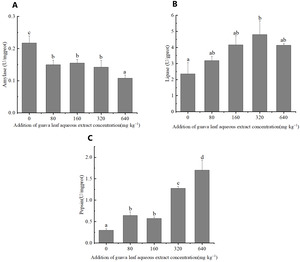
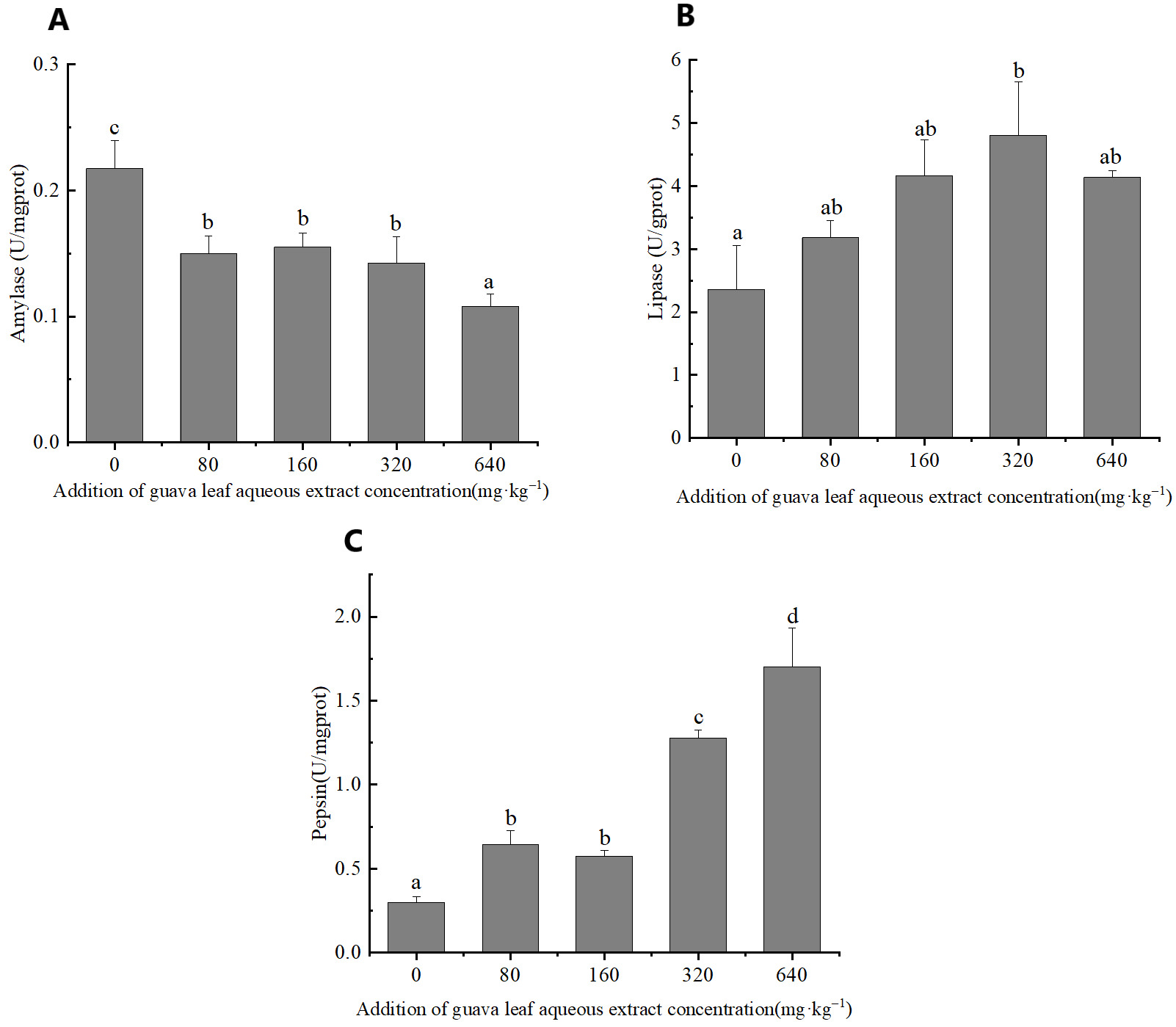
Digestive enzymes activities
The feeding of guava-leaf extract affected digestive enzyme activity in the gastric tissues of mud crabs. The AMS activity of the experimental groups was significantly decreased compared to the control group (P < 0.05) (Figure 1). The pepsin activity in gastric tissues increased with the increase in the level of guava-leaf extract in the feed, and the pepsin activity in the 320 mg·kg–1 and 640 mg·kg–1 guava-leaf extract groups was significantly decreased compared to the control group (P < 0.05) (Figure 1). Lipase activity in gastric tissues tended to increase and then decrease with increases in the levels of guava-leaf extract in feed. Lipase activity in the 320 mg·kg–1 guava-leaf extract group was significantly higher than that in the control group (P < 0.05) (Figure 1).
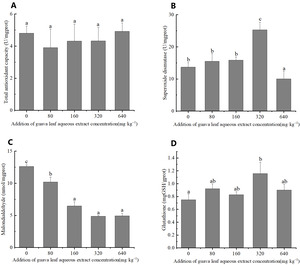
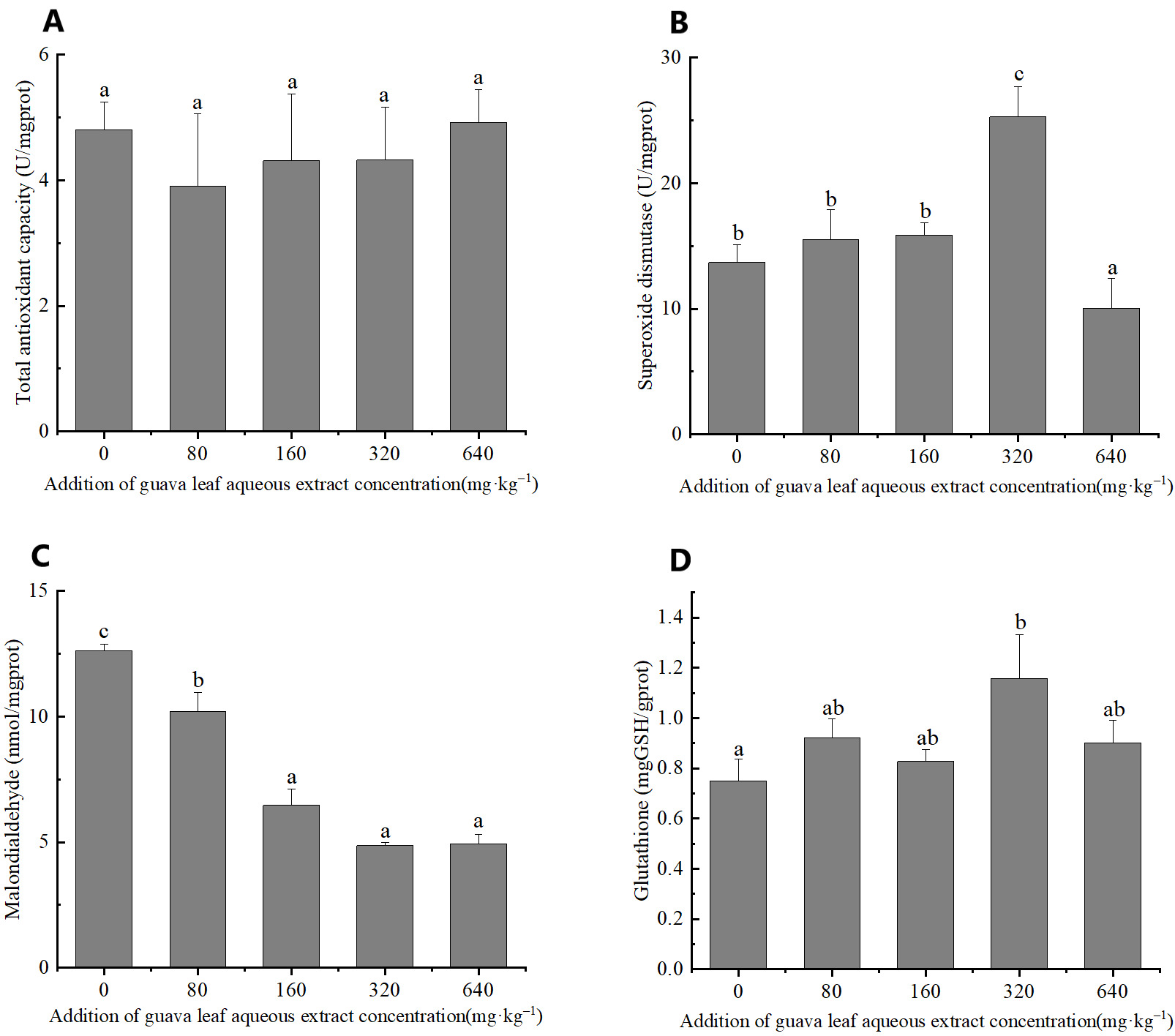
Antioxidant enzymes activities
The results of one-way ANOVA indicated that the addition of different amounts of guava-leaf extract to feed had significant effects on SOD activity, and MDA, and GSH content in mud crabs (P < 0.05), but not on T-AOC (P > 0.05) (Figure 2). SOD activity was significantly higher in the 320 mg·kg–1 guava-leaf extract group than the control group (P < 0.05) and significantly lower in the 640 mg·kg–1 guava-leaf extract group (P < 0.05) (Figure 2). MDA activity was significantly decreased in the 160 mg·kg–1, 320 mg·kg–1, and 640 mg·kg–1 guava-leaf extract groups compared to the control group (P < 0.01), and significantly decreased in the 80 mg·kg–1 guava-leaf extract group (P < 0.05) (Figure 2). T The GSH levels in hepatopancreas tissue were significantly higher in the 320 mg·kg–1 guava-leaf extract group than in the control group (P < 0.05) (Figure 2).
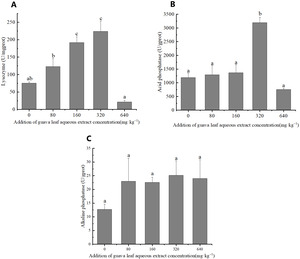
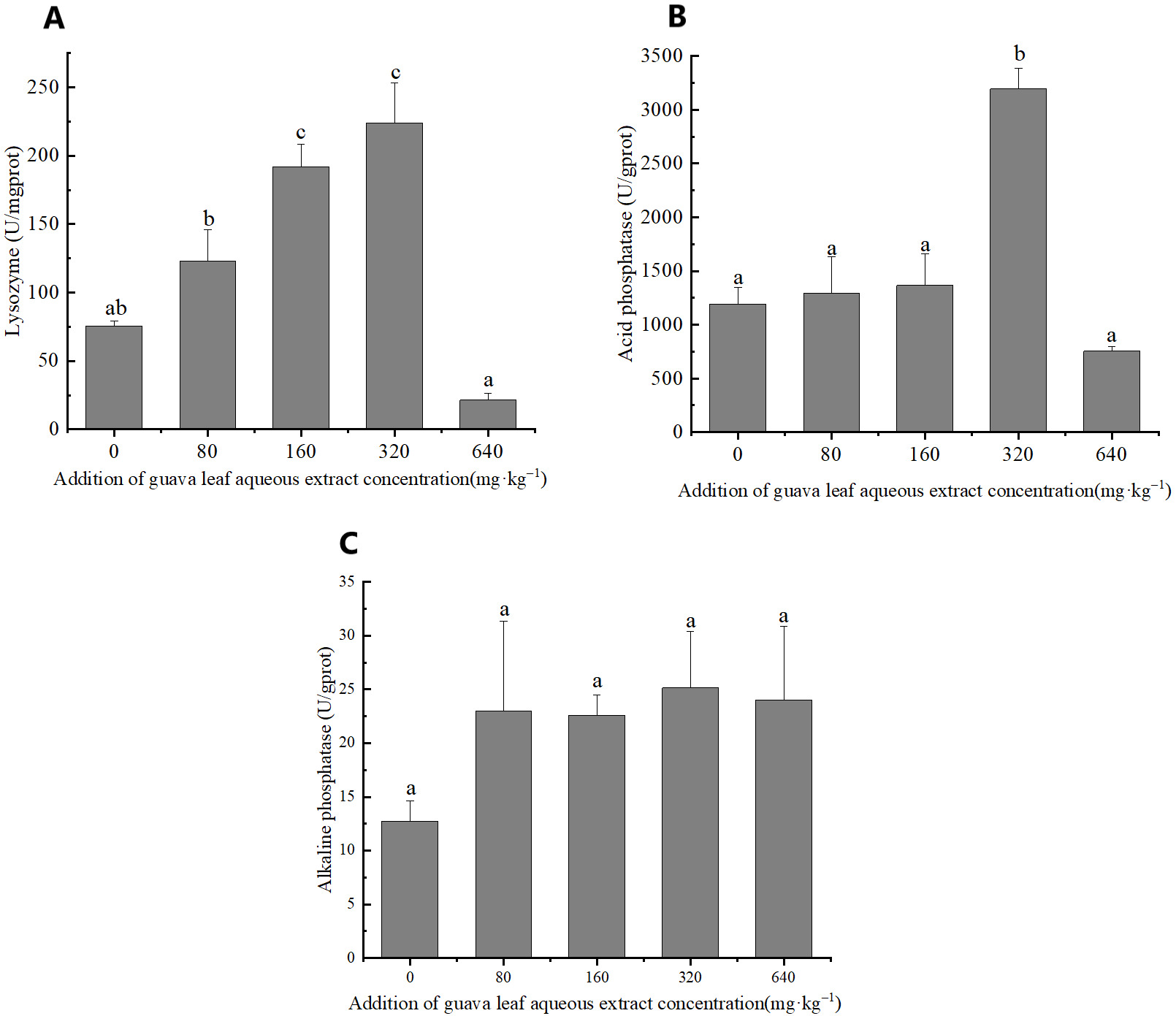
Immunoenzymes activities
The results of one-way ANOVA showed that the addition of different amounts of guava-leaf extract to feed had a significant effect on LZM and ACP activities in mud crabs (P < 0.05), but not on AKP activity (P > 0.05) (Figure 3). LZM activity was significantly elevated in the 160 mg·kg–1 and 320 mg·kg–1 guava-leaf extract groups compared to the control group (P < 0.05) (Figure 3), while ACP activity was significantly elevated in the 320 mg·kg–1 guava-leaf extract group (P < 0.05) (Figure 3).
Gene expression
The expression of the relevant genes was measured and summarized in Figure 4. The level of GPx3 gene expression in hepatopancreatic tissue showed a trend of increasing and then decreasing with increasing amounts of guava-leaf extract in the feed. The 80 mg·kg–1 guava-leaf extract group was also upregulated compared to the control group (P < 0.05). The expression levels of the CAT gene were elevated in the experimental groups compared to the control group, with expression in the 80 mg·kg–1 and 640 mg·kg–1 guava-leaf extract groups being significantly higher than the control group (P < 0.05). The expression levels of JNK genes were elevated in the experimental groups compared to the control group, with the 80 mg·kg–1 guava-leaf extract group being significantly higher (P < 0.05). The expression levels of SOD genes in hepatopancreatic tissues showed a trend of increasing and then decreasing, which was consistent with the SOD activity levels. All experimental groups exhibited higher levels of SOD expression compared to the control group, with the levels in the 80 mg·kg–1, 160 mg·kg–1, and 320 mg·kg–1 guava-leaf extract groups significantly increased (P < 0.05). The expression levels of GST and P53 genes were significantly up-regulated in all experimental group compared to the control group (P < 0.05).
Discussion
Chinese herbs are widely used in aquaculture as alternative functional additives to antibiotics because of their low cost and non-toxicity, numerous studies have demonstrated that Chinese herbs can promote the growth, digestive enzyme activity, immune response, and antioxidant capacity of aquatic animals.18 The addition of Astragalus and Wolfberry to feed can enhance the growth of grass carp and tilapia.19 Similar to the findings of previous studies, the SR of mud crabs in all experimental groups in this study was higher than that of the control group. The SR of the 320 mg·kg–1 guava-leaf extract group was 30% higher than the control group. The WG and SGR of the 320 mg·kg–1 guava-leaf extract group were increased compared to that of the control group. Thus, guava leaf aqueous extract exhibited a growth-promoting effect on mud crabs when added to feed.
Guava-leaf extract contain guava leaf polyphenols, which regulate the absorption of dietary carbohydrates in the intestine.20 In this study, lipase and pepsin activities in the gastric tissue of mud crabs were increased in all experimental groups (Figure 1). The addition of guava leaf aqueous extract to feed could promote the digestion of mud crabs. However, Chinese herbal medicine does not improve digestion were also reported. Dietary Astragalus polysaccharides, chlorogenic acid, and allicin failed to enhance digestion of Litopenaeus vannamei.21 Dietary medicinal herbs also failed to significantly improve the growth of juvenile red sea bream Pagrus major.22 Nevertheless, other aspects performance of animals in those two experiments was enhanced to different degrees. It was speculated that those discrepancies were due to the difference in CHM, which would impact another aspect of the body and not just growth performance.23
The antioxidant system of an organism scavenges excess reactive oxygen species from the body and provides protection from oxidative damage. Dietary supplementation of Chinese herbs and probiotics can improve the antioxidant parameters of Nile tilapia.24 Similarly, dietary supplementation of Astragalus polysaccharide, chlorogenic acid, and allicin can increase the antioxidant capacity of Litopenaeus vannamei.25
Guava-leaf extract exhibit antioxidant activity and are a potent source of natural antioxidants. In this study, the antioxidant capacity of hepatopancreas tissue from mud crabs fed guava-leaf extract was assessed. The SOD activity and GSH content in all experimental groups were significantly higher than those in the control group, and exhibited a trend of first increasing and then decreasing as the amount of guava leaf aqueous extract increased. SOD is the important enzymes in the antioxidant system. In the present study, the expression of the SOD gene exhibited the same trend as SOD activity. The expression levels of GPx3 and GST genes of all experimental groups were also higher than those of the control group. The sulfhydryl groups of GSH, GST, and GPx3 are active groups that readily bind to specific drugs and toxins, enabling them to have detoxifying effects.26 The MDA content of all experimental groups was significantly decreased compared to that of the control group. MDA content can reflect the degree of lipid peroxidation in the body. Those results suggest that guava leaf aqueous extract can promote the regulation of antioxidant function in the hepatopancreas of mud crabs. Similarly, feeding guava-leaf extract to spot prawns increased SOD activity, decreased MDA levels and increased total antioxidant capacity.11 Some studies also demonstrated that guava-leaf extract increased the activities of antioxidants and immune-related enzymes in mud crab.
The non-specific immunity of animals is closely related to their health and directly affects their SR and health of animals. Many studies have also shown that herbs can improve the immunity of aquatic animals.27 The addition of Huanglian Jiedu’s decoction to feed increased resistance to lactococcal disease in oregano (Choi et al., 2014). Adding Huanglian Jiedu’s decoction to feed increased resistance to lactococcal disease in the oregano (Choi et al., 2014a). Similarly, adding Astragalus and Huanglian to feed increased the bactericidal activity of grass carp. Chinese herbs also had a healing effect on the traumatic surface of the body wall of sea cucumber.28 Guava leaf aqueous extract contain compounds that stimulate immune activity29 and the use of guava-leaf extract in crab breeding can significantly improve the immune capacity of crabs.
In the present study, ACP and LZM activities were significantly higher in all experimental groups than in the control group. JNK and P53 expression levels were increased compared to the control group. Thus, those results indicate that guava-leaf extract could improve the immunity of mud crabs. ACP enhances the recognition and phagocytosis of foreign bodies by blood cells and is the material basis for pathogen killing.30 Previous studies have shown that Chinese herbs can activate nonspecific immunity by increasing lysozyme activity and accelerating phagocytosis.31 LZM is also important to the innate immune system of fish. A previous study showed that the addition of guava-leaf extract to feed can improve the immunity of spot prawns,11 and Oreochromis mossambicus.10
Conclusion
In conclusion, the addition of guava leaf aqueous extract to mud crab feed improved growth, digestion, non-specific immune function, and antioxidant capacity. The addition of 320 mg·kg–1 guava-leaf extract was optimal in terms of SR, WG, SGR, SOD, GSH, MDA, ACP, LZM, and CAT, SOD, GST, P53 in this study. Therefore, the recommended level of guava leaf supplementation in mud crab feed is 160-320 mg·kg–1.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Key-Area Research and Development Program of Guangdong Province (2021B0202040001), the China Agriculture Research System of MOF and MARA (CARS-48), the Rural Science and Technology Correspondent Fund of Guangzhou (20212100051), and the Guangzhou Municipal Science and Technology Project (202206010138).
GRediT - Authors’ Contributor Roles
Conceptualization: Yan Yue (Equal), Zhi-Xun Guo (Equal). Methodology: Yan Yue (Lead), Hong-Ling Ma (Equal), Chang-Hong Cheng (Equal), Guang-Xin Liu (Equal), Zhi-Xun Guo (Lead). Formal Analysis: Yan Yue (Equal), Guang-Xin Liu (Equal). Writing – original draft: Yan Yue (Lead). Writing – review & editing: Yan Yue (Equal), Zhi-Xun Guo (Equal). Funding acquisition: Si-Gang Fan (Equal), Zhi-Xun Guo (Lead). Resources: Jian-Jun Jiang (Supporting), Zhi-Xun Guo (Lead). Supervision: Zhi-Xun Guo (Lead).