Introduction
Aquaculture includes species such as micro-macro algae, fish, mollusks, and crustaceans naturally found in seas and inland waters and produced in facilities, and the nutrients obtained from them.1 Aquaculture is an industry that continues to grow worldwide. Aquaculture studies in Turkey are rapidly increasing their share in aquaculture production every year, and aquaculture production has increased almost 12 times in the last 30 years, with an annual average increase of 8.8% worldwide.2 While Turkey’s fishing production fluctuates over the years, aquaculture production continuously increases. While trout, carp, and eel farming are carried out in the inland waters of Turkey, sea bream and sea bass are grown in the seas. Live foods are essential in aquaculture, especially in the larval stages of some economic marine fish such as sea bream, sea bass, turbot, and coral. In marine fish farming, the quality of the first live bait used immediately after the larvae open their mouths is essential in increasing their survival and growth rates. Microalgae production is important in Turkey’s larval breeding of sea bream and sea bass.3
In terms of their external appearance, algae are mostly eukaryotic and photosynthetic organisms that can be observed in different morphologies from single-celled and filamentous microscopic forms to plants several meters tall, have a cellulose wall, have a simple structure, do not show true roots, stem, and leaf structure, and have a thallus structure. Thanks to the pigments in their structures, algae transform carbon dioxide and water into carbohydrates with the effect of light, thereby increasing the nutritional value in the aquatic environment and forming the food source of many aquatic creatures. It also increases the dissolved oxygen ratio in the water.4
Algae are one of the most important producers of the food chain. The interest in algae is increasing because they are generally used as food in the Far East. Algae can be an important source in meeting nutritional needs in the future due to reasons such as increasing their weight 2-3 times a day under suitable conditions, being easy to produce, being economical, and having no side effects.4 Algae have been used for various purposes in many areas from past to present.5 Agriculture, animal nutrition, waste treatment, aquaculture, cosmetics, biodiesel production, medicine, and pharmacy are the areas where algae are widely used.2,6
Algae come to the forefront as sources suitable for use as food and foods due to their high content of components such as protein, polysaccharides, lipids, vitamins, minerals, amino acids, fatty acids, carotenoids, and the bioactive components they produce.7
Microalgae have particular importance in aquaculture to provide zooplankton nutrition in larval feeding. For this reason, the size of algae and zooplankton and the amount of nutrients is essential. Species produced for Rotifer or Artemia feeding are Chlorella sp., Chlamydomonas sp., Nannochloris oculata, Nannochloropsis oculata, Tetraselmis tetrathele, and Tetraselmis chuii. Nannochloropsis sp. It feeds the larvae for rotifer food and green water technique in fish breeding facilities. Rotifers are one of the most widely used live foods because of their high reproduction and survival rate, easy digestion by larvae, and low production cost.8 In addition, rotifers are also called live capsules because they provide the necessary nutrients for the healthy development of the larvae.9 Algae, usually produced in fish hatcheries, to feed rotifers or Artemia; Chlorella sp., Chlamydomonas sp., Nannochloris oculata, Nannochloropsis oculata, Tetraselmis tetrathele and T. chuii.10 Nannochloropsis sp. is mainly used in fish farms to feed fish larvae for rotifer food and green water technique. Microalgae are used as live feed for the cultivation of bivalve mollusks (such as oysters, mussels, and scallops), some marine gastropod larvae (such as abalone), shrimp larvae (Penaeus and Metapenaeus), some fish species (such as tilapia, Silver Carp) and also zooplankton—usually Chlorella sp., Isochrysis sp., and Nannochloropsis sp. Microalgae species are rich in some fatty acids, minerals, and vitamins and are used as enrichment in the nutrition of zooplankton, such as rotifers. Microalgae generally contain 30-40% protein, 10-20% lipid, and 5-15% carbohydrates in the last logarithmic growth stage.11 Nutritionally, live microalgae are more nutritious and digestible than other alternative foods. The quality of food sources depends on many chemical components such as PUFA, vitamins, sterols, and carbohydrates.12
The use of phytoplankton in sea bream farming is a financial necessity. Although microalgae have a positive role in rearing fish and shrimp larvae, the reason is still unknown. In addition, some microalgae species contain fatty acids that play a significant role in the development and survival of fish larvae, such as docosahexaenoic acid (DHA; 22:6ω3), eicosapentaenoic acid (EPA; 20:5ω3), arachidonic acid (ARA; 20:4ω6). In addition, they are rich in essential fatty acids.13 The use of phytoplankton in sea bream farming is a financial necessity. Although microalgae have a positive role in rearing fish and shrimp larvae, the reason is still unknown. In addition, some microalgae species contain fatty acids that play a significant role in the development and survival of fish larvae, such as docosahexaenoic acid (DHA; 22:6ω3), eicosapentaenoic acid (EPA; 20:5ω3), arachidonic acid (ARA; 20:4ω6). In addition, they are rich in essential fatty acids.13 They cultured a helical photobioreactor illuminated by continuous artificial light in an F/2 medium of Nannochloropsis sp.. When the nutrient composition was examined, it was determined that the lipid profile included mono and polyunsaturated fatty acids with high nutritional value. Among them, an EPA amount of more than 2% is striking. Other predominant fatty acids are palmitic acid, palmitoleic acid, and to a lesser extent, oleic acid. Ashour and El-Wahab14 cultivated N. oceanica in culture media with different nitrogen/phosphorus ratios (N:P) and found that the lipid content of the biomass increased as this ratio decreased. At the same time, the biomass of the crop decreased. Olofsson et al.15 studied the same problem in N. oculata and found an increase in biomass lipid content and a decrease in biomass after lowering the N:P ratio. Nannochloropsis sp. Although microalgae are rich in omega-3 fatty acids, approximately 37.6% (by weight) carbohydrates, 28.8% protein, 18.4% total fat 3% microelements (Na, P, K, Ca, Mg, Zn, Fe) were also reported to contain.16 The use of phytoplankton in sea bream farming is a financial necessity. Although microalgae have a positive role in rearing fish and shrimp larvae, the reason is still unknown. In addition, some microalgae species contain fatty acids that play a significant role in the development and survival of fish larvae, such as docosahexaenoic acid (DHA; 22:6ω3), eicosapentaenoic acid (EPA; 20:5ω3), arachidonic acid (ARA; 20:4ω6). In addition, they are rich in essential fatty acids.13
They cultured a helical photobioreactor illuminated by continuous artificial light in the F/2 medium of Nannochloropsis sp. When the nutrient composition was examined, the lipid profile revealed mono and polyunsaturated fatty acids with high nutritional value found to contain. Among them, an EPA amount of more than 2% is striking. Other predominant fatty acids are palmitic acid, palmitoleic acid, and to a lesser extent, oleic acid. Ashour and El-Wahab14 cultivated N. oceanica in culture media with different nitrogen/phosphorus ratio (N:P) and found that the lipid content of the biomass increased as this ratio decreased. At the same time, the biomass of the crop decreased. Olofsson et al.15 studied the same problem in N. oculata and found an increase in biomass lipid content and a decrease in biomass after lowering the N:P ratio. Nannochloropsis sp. Although microalgae are rich in omega-3 fatty acids, approximately 37.6% (by weight) carbohydrates, 28.8% protein, 18.4% total fat 3% microelements (Na, P, K, Ca, Mg, Zn, Fe) were also reported to contain (Olofsson et al.15
For successful production in larval breeding, high-quality, easily digestible, vital nutrients that provide optimum growth should be used.17 Many fish species die rapidly in early development due to poor quality and suitable feed. Previous studies on larvae have shown that live baits provide a higher survival rate than microparticles. Live-feed organisms used in aquaculture are rotifer, cladoceran, artemia, and copepod species.18
Various factors affect the development of phytoplankton. Salinity is one of the most critical environmental factors affecting phytoplankton development, metabolism, and distribution. Depending on the increase in salinity, the photosynthesis and protein synthesis capacities of the cells decrease; On the other hand, it has been reported that microalgae transfer their rich fatty acid content to the larva via rotifers, increasing the lipid ratios and decrease the growth rate, improving the survival rate and growth of the larvae.19 Significant losses are observed in production due to the further increase in salinity.20
In this study, the green microalgae N. oculata (Droop) algae, widely used in rotifer feeding in the Turkish aquaculture production process, was used to examine the effects of salinity on phytoplankton. Cell growth and specific growth rate of N. oculata were determined continuously at 30‰ salinity in a helical photobioreactor.
Materials and Methods
Materials
The experiment was carried out in the Plankton laboratory of Mersin University Faculty of Fisheries. Nannochloripsis oculata, used as a trial material, was obtained from the Çukurova University Faculty of Fisheries (Figure 1). Conway medium21 was used as an algae nutrient medium. Sea water used in the culture medium of N. oculata algae was first passed through a 25 μm and then a 1 μm cartridge filter. Then it was used in the bioreactor after being passed through UV.
The study was carried out by repeating three times at 30‰ salinity in the N. oculata helical photobioreactor. N. oculata was produced for two weeks in a helical photobioreactor with a continuous production system, and daily cell increase and growth rate were tried to be determined. During the experiment, daily counts of N. oculata algae and daily cell density and growth rate were determined.
Calculation of Cell Increase and Growth Rate of Nannochloropsis aculat algae
Daily counts were made on a Neubauer counting camera.22 To ensure the continuity of the culture, which reached the maximum growth rate at the same growth rate, it was taken into production in the continuous culture system. In the system, nutrient-enriched seawater was added to the algae container continuously and constantly, and the algae were harvested by removing the added amount from the environment. Thus, the cell concentration in the culture dish did not change with time and remained constant. The nutrient-enriched seawater’s entry rate was regulated by the species-specific growth rate (µ) and the residence time of the cells in the medium.
Dilution rate (D); It is expressed as the flow rate ratio to the culture volume.23
D=µ=XAK/XKV
D= dilution rate, XAK= flow rate, XKV= culture volume
After the algae, initially inoculated into the culture water, reached the maximum density, its production was continued in the bioreactor with a continuous culture system. The algin dilution rate was carried out by providing the flow rate appropriate to the specific growth rate of the algae. The specific growth rate (μ) of N. oculata was determined according to the equation in the experiment. Here, μ represents the growth rate per unit amount.24,25
Specific Growth Rate (K=µ= division/day),
K= µ = LnNt -Ln No/ t1-t0
K: growth rate,
Nt: cell number at the end of the experiment,
N0: initial cell number,
T: time (day)
The average initial density in the experiment was determined as 10.26x106. 4.5 L algae portion of the stock culture obtained by batch method at 30‰ nutrient ratio was inoculated into the culture water of the 20 L stock container in the helical system. The total volume of the culture suspension was determined as 24.5L. The helical photobioreactor of the system consists of a transparent hose with an inner diameter of approximately 1.8 cm, a length of 95 m, and a volume of 27 L
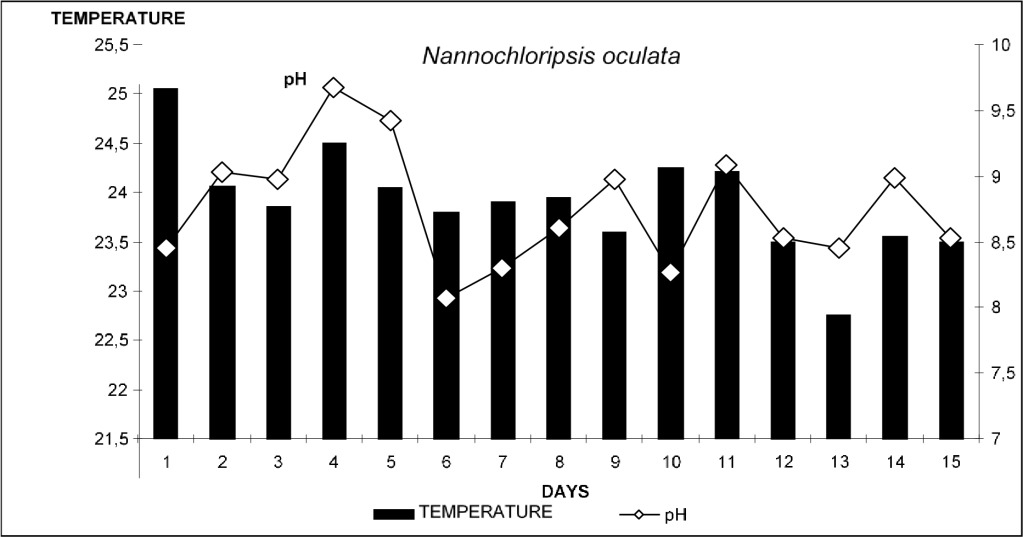
The 15L alga was harvested with a dilution rate of 10.4 mL/min and added to the 15L nutrient culture suspension with the same flow rate every day. The temperature was determined during the experiment between 23.0–24.8°C and pH 7.32–9.91 (Figure 2). During the lighting experiment, 2000 lux light intensity was provided by four 40 W daylight fluorescent lamps continuously.
Bioreactor Designed in Experiment
A helically designed photobioreactor was used under laboratory conditions (Figure 3). A peristaltic pump was used to circulate the algae in the helical bioreactor. The system was carried out indoors by utilizing an artificial light source.
In the helical system, adding a container in which algae could be collected to the system was deemed appropriate. The freshly prepared nutrient was added with the same flow rate to the algae harvest container where the algae were collected. For the ideal growth of N. oculata alga, the pH has been balanced in the range of 7.32–9.91. To balance the pH in the system, carbon dioxide was added to the environment at regular intervals (1‰). The helical photobioreactor used in the experiment; is designed for continuous production under laboratory conditions. According to the statistical data in the experiment; the SPSS (V.26) program27 was used in the statistical evaluation of the findings collected during the study.28 In the experiment, the cell number of the algae was examined for 15 days. While there was no difference between measurements on the same day, the difference between days in terms of algal cell count was significant for all days (P<0.05).
Results
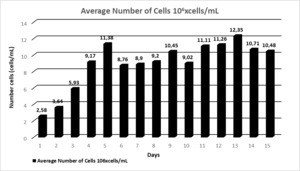
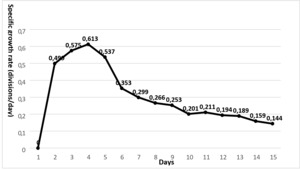
The initial average planting density of N. oculata is 1.068x106 at 30‰ salinity concentration. The number of cells of N. oculata increased logarithmically between 1–5th days. Cell density, the highest mean cell increases on day 5 was 11.38x106±0.335 cells/mL; the highest mean specific growth rate was 0.613±0.056 divisions/day on the 4th day and 0.537±0.046 divisions/day on the 5th day. Average cell increases 11th,12th, and days 13th, 11.11x106±0.338 cells/mL, 11.26x106±0.827 cells/mL, 12.35x106±0.165 cells/mL, respectively; mean specific growth rates were determined as 0.211±0.012 divisions/day, 0.194±0.018 divisions/day and 0.189±0.016 divisions/day, respectively. The maximum mean cell increase was 12.35x106±0.165 cells/mL on the 13th day; the mean specific growth rate decreased by 0.189±0.016 divisions/day (Table 1; Figure 4,5).
Cell increases and specific growth rates at 30‰ salinity concentrations were investigated in the N. oculata algae species helical photobioreactor, which is widely used in rotifer feeding in our country.
Discussion
In the study, the highest cell number in the N. oculata species was 11.38x106±0.335 cells/mL on the 5th day at 30‰ salinity concentration. It was determined as 12.35 x106±0.165 cells/mL on the 13th day. The highest growth rate in this species was determined as 0.189±0.016 division/day on the 13th day at ‰30 salinity concentration.
Abu-Rezq et al.24 Nannochloropsis sp. reported that the maximum cell number was 24.9-32.4x106 cells/mL, and the growth rate was 0.11-0.13 divisions/day at 20-40‰ salinity concentrations. The highest growth rate values detected in our study, Abu-Rezq et al.,24 coincide with their work. However, when the results obtained in all experimental groups in another study were examined, it was stated that the cell density was better at low salinity in those fed with D. tertiolecta and I. galbana. It was determined that T. suecica, N. oculata, and Chlorella sp. algae gave better results in terms of cell densities at high salinity.29 In algae, it was determined that it gave better results regarding cell densities at high salinity. This shows the limiting effect of different nutrients on growth rate. According to another researcher, It has been stated that N. oculata is tolerant to different salinity ranges (0-40%) experimentally. It was determined that the cell density and dry weight increased from 17.1 ± 0.4 to 26.6 ± 0.1 ml-1 and from 6.4 ± 0.1 to 9.7 ± 0.3 mg-1, respectively. However, it was stated that the growth rate regressed under 40% to 0% salinity stress. Accordingly, it has been reported that the cell density for N. oculata algae is best at 30%0 salinity. Many halotolerant microalgae have a response mechanism that allows them to exist in a salty environment. These strains produce metabolites that help protect them from salt injury and maintain an osmotic balance between the cell and its environment.30 In our study, while the cell density of N. oculata species increased at ‰30 salinity concentrations, their growth rate decreased.
In this study, Nannochloropsis sp. Although the best salinity for algae was defined as 30%0, the best salinity that increased oil formation in N. oculata caused a decrease in cell number.31 Again, a researcher reported that the salinity concentration for Nannochloropsis species varies between 20-40‰ under optimum production conditions.24 Another researcher stated the optimum salinity concentrations for Nannochloropsis oculata species as 28‰ and 4–36 ‰, respectively.32 In our study, the salinity concentration at which the highest cell numbers were detected was close to the optimum values determined by various researchers.
As a result, adjusting the salinity concentration in the range of 25-30‰ in N. oculata cultures to increase the number of algal cells and achieve a higher growth rate can be recommended. Photobioreactor design in microalgae biotechnology is essential both scientifically and economically. Photobioreactor design aims to make new culture systems for optimal use of high-level light sources such as solar energy.33 However, using artificial lighting in the commercial production of photosynthetic cells increases the costs of algal biomass. Also, in their Nannochloropsis sp. study, Konuralp et al.34 stated that this species needs medium temperatures. Still, it quickly becomes polluted at high and low temperatures, so it is an alternative species for cultivation in closed environments.
In our study, N. oculata species were grown in a continuous system in a helical photobioreactor at 30‰ salinity, and the seeding density was determined as 1.068x106±0.006 cells/mL. In the experiment, N. oculata was produced for two weeks, and align cell growth increased logarithmically during the first five days. From the 5th to the 15th day, the cell increase was determined as 12.35x106±0.165 cells/mL at the highest and 8.76x106±0.709 cells/mL at the lowest. However, on the 13th day, it was determined that the increase in algin cells was 12.35x106±0.165 cells/mL, and the specific growth rate decreased by 0.189±0.016 divisions/day. It was determined that the growth of algae cells increased 10 times between 5-15th days of planting density. As Renaud and Parry35 stated, it was concluded in our study that photobioreactor designs are essential scientifically and economically and that algae production in the continuous system increases algae yield in culture. According to statistical data, the cell growth of algae was investigated for 15 days in the experiment. While no difference was observed between daily measurements (P>0.05), the difference between days in terms of algal cell count was significant for all days (P<0.05).
Environmental factors (such as temperature, light, pH, and salinity) affect algae production conditions. Increased water temperatures from global warming can lead to increased salinity by excessive evaporation in the open algae production system. The cells ’ photosynthesis and protein synthesis capacities decrease depending on the increase in salinity. With further increase in salinity, a significant limiting effect on the growth rate and the final product occurs. High salinity creates physiological stress on microalgae and causes lipid accumulation. Therefore, halo-stress can increase lipid accumulation in microalgae for biodiesel production. However, while halo stress increases the amount of lipids in algae, a significant decrease in cell division is observed. Nannochloropsis are marine algae Eustigmatophytes, known as good lipid producers and fast-growing microalgae. In addition, Nannochloropsis sp. algae tolerate many environmental stresses, including salinity.31,36
In this study, the low growth rate of N. oculata algae at 30‰ salinity concentration showed limiting effects on algae. It is known that nitrogen sources and different concentrations are effective in the growth and biochemical structures of microalgae. Ashour and El-Wahab14 cultured N. oceanica in culture media with different nitrogen/phosphorus ratios (N:P) and found that the lipid content of the biomass increased as this ratio decreased. At the same time, they noted that the primary productivity of the crop was reduced. Another investigator reported an increase in the lipid content of biomass and a decrease in biomass in N. oculata after lowering the same N:P ratio.15 It is recommended to develop research in this direction and to carry out more comprehensive studies on the effects of different salinity rates on algae’s cell density and growth rate. In addition, the effects on the biochemical content of algae should also be examined.
As a result, the cell numbers of N. oculata algae are 5-15. increased 10 times between days. According to the research results, N. oculata algae were produced in the helical bioreactor for long periods, and algae product was obtained at maximum cell density.
At half the reactor volume, N. oculata algae were harvested daily in the helical bioreactor. Thus, high-quality production was achieved in the culture of N. oculata algae, and it was possible to predict the amount of algae cultured in the helical photobioreactor in advance.
Author contributions
Conceptualization: Hilal Kargin (Lead). Investigation: Hilal Kargin (Lead). Data curation: Hilal Kargin (Lead). Formal Analysis: Hilal Kargin (Lead). Writing – original draft: Hilal Kargin (Lead). Writing – review & editing: Hilal Kargin (Equal).