Introduction
According to the FAO statistics on aquaculture and fisheries, European catfish, a species native to central and eastern Europe, is distributed in 47 countries today, whether for natural dispersal or culture transport. Although the interest in European catfish culture has increased, studies on the culture method, immunity, and treatment of diseases of the species have been very limited.1 In European catfish breeding, there is much loss due to parasitic diseases at the larval stage.2 Ichthyophthirius multifiliis, commonly known as “Ich,” “white spot disease,” or “ichthyophthiriasis,” is a parasitic ciliate protozoan that affects European catfish. The parasite attaches to the fish skin and gills of the fish, causing irritation and damage, and can ultimately lead to death if left untreated.3 The life cycle of Ich begins with the release of motile infective stages, known as theronts, into the water. The theronts can infect a wide range of intermediate hosts, including crustaceans, copepods, and fish. Once inside the host, the theronts transform into a parasitic stage called a trophont. Following a period of growth and development, it releases the host and turns into an encysted tomont. The tomonts then divide and form hundreds of small tomites, released into the water as theronts to infect the definitive host, starting the cycle over again.4,5
The parasite causes significant economic losses in the aquaculture industry, making the development of effective treatments crucial. The treatment of Ich in fish is typically done through antiparasitic drugs, such as malachite green, formalin, hydrogen peroxide, peracetic acid, sodium percarbonate, humic acid, bronopol, and potassium ferrate.6 These treatments can be effective if used correctly, but it is essential to remember that Ich is a highly resilient parasite and can persist in the environment for long periods. This makes it challenging to eradicate Ich from an infected system entirely, and it is common for fish to become reinfected even after treatment. Although malachite green is also widely used to control I. multifilis, it has been determined to be bio-accumulating in the ecosystem and fish tissues and also has cytotoxicity, carcinogenicity, and mutagenicity effects.7 Therefore, its use within the European Union was banned in 2000 under EC directive 90/676/EEC; regulation 2377/90/EEC as a result of the widespread prohibition on the use of malachite green, extensive research needs to be on providing alternative effective and environmental-friendly products for the control of I. multifiliis infections over the past few decades.
Medicinal plants have been shown to possess antiprotozoal activity, and their use in traditional medicine has led to the development of new antiparasitic drugs. Unlike conventional chemical treatments, medicinal plants are generally considered safe for the environment and do not pose a risk to human health. Additionally, many medicinal plants are readily available and inexpensive, making them an attractive option for aquaculture producers. In this study, antiparasitic effects of the essential oils (onion (A. cepa), sage (S. officinalis), menthe (M. spicata), garlic (A. sativum), lavender (L. officinalis), and oregano (O. onites) evaluated against I. multifiliis trophonts. This is the first report on the antiparasitic effect of these essential oils against I. multifiliis protozoan parasite.
Materials and Methods
Fish
Heavily infected with I. multifiliis European catfish weighing 29.38±2.24 g (mean ±SD) were obtained from Bursa (in Turkey). All fish were kept in 80 L fiberglass tanks with a recirculating aquaculture system (RAS) containing mechanical, biological, and ultraviolet (UV) filters at the Fisheries Research and Application Unit of the Fisheries Faculty of Isparta Applied Science University, Turkiye. The water quality was measured at a temperature of 18.5±0.2°C, dissolved oxygen of 6.0 mg L-1, and pH 7.3. A total of 168 infected European catfish were anesthetized (clove oil; 50 mg L-1) and examined for the prevalence and intensity of the parasite under a light microscope (Olympus) at 100× magnification before the experiment.8 The infection rate was 100%, and the mean number of parasites on the gills and skin was 20-90 individuals per fish.
Plant essential oil
Onion (A. cepa), sage (S. officinalis), menthe (M. spicata), garlic (A. sativum), lavender (L. officinalis), and oregano (O. onites) essential oils were obtained from a commercial company (Manolya Natural and Aromatic Products Ltd., Isparta).
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
Chemical components of plant essential oils were determined as percentages using GC-MS. The GC-MS analyses were performed using a Hewlett-Packard 6890 series gas chromatograph (Perkin Elmer (PE) Auto System XL, USA) equipped with the capillary column, CPWax 52 CB (50 m 9 0.32 mm; film thickness ¼ 0.25 lm). The operating conditions were as follows: the GC temperature program was held at 60-220°C for 2 min and then at 220°C for 20 min. The carrier gas was helium; the flow rate was 40 mL min-1; the injector and detector temperature was 240°C; the sample injection volume was 1 μL; and the split ratio was 1/20 mL min-1. Relative percentage amounts were calculated from chromatograms by the Turbo Crom.
In vitro antiparasitic activity
The skin and gill arches of infected fish were scraped into a sterile petri dish containing 2 mL of distilled water, and active moving trophonts were removed with forceps. In vitro tests were conducted on a 96-well plate. First, the essential oils were dissolved in dimethyl sulfoxide (DMSO) to prepare in the different concentrations for testing the solutions (0.1, 0.25-, and 0.5-mL L-1). Then, 100 μL water with 90 trophonts and 100 μL essential oil solutions were added, each well, and incubated the parasites for 5, 30, and 60 minutes. The trophonts were examined under the microscope (× 100). The immobilized and lysed trophont cells were considered as dead.9 Sterilized distilled water containing DMSO was used for the negative control groups, while the positive control groups were prepared by using formaldehyde (0.1 mL L-1) exposed for 30 minutes. All the tests were performed in triplicate.
Statistical analysis
All experimental data were expressed as mean ± SD (standard deviation) and evaluated using the SPSS 19.0 statistical package program (SPSS Inc, Chicago, IL, USA). Statistical significance was determined by variance (one-way ANOVA) and multiple comparison test (Duncan) at p<0.05.
Results
Gas Chromatography-mass spectrometry (GC-MS) analysis of essential oils
According to GC-MS results of essential oil, the major components are dipropyl disulfide (46.15%), dipropyl trisulfide (14.10%), and 1-propenyl propyl disulfide (5.14%) in onion (A. cepa); α-thujone (46.80%), (E)-β-caryophyllene (7.47%), camphor (12.14%), 1,8-cineole (5.80%), and β-pinene (4.35%) in sage (S. officinalis); carvone (48.00%), and limonene (12.60%) in menthe (M. spicata); diallyl disulfide (32.70%), diallyl trisulfide (18.00%), allyl methyl trisulfide (6.26%), diallyl sulfide (4.24%), and diallyl tetrasulfide (4.11%) in garlic (A. sativum); 1,8-cineole (32.65%), linalyl acetate (22.90%), linalool (15.00%) and borneol (4.30%) in lavender (L. officinalis); carvacrol (73.00%), thymol (14.40%), linalool (5.60%) in oregano (O. onites) (Table 1).
In vitro antiparasitic efficacy of the essential oils
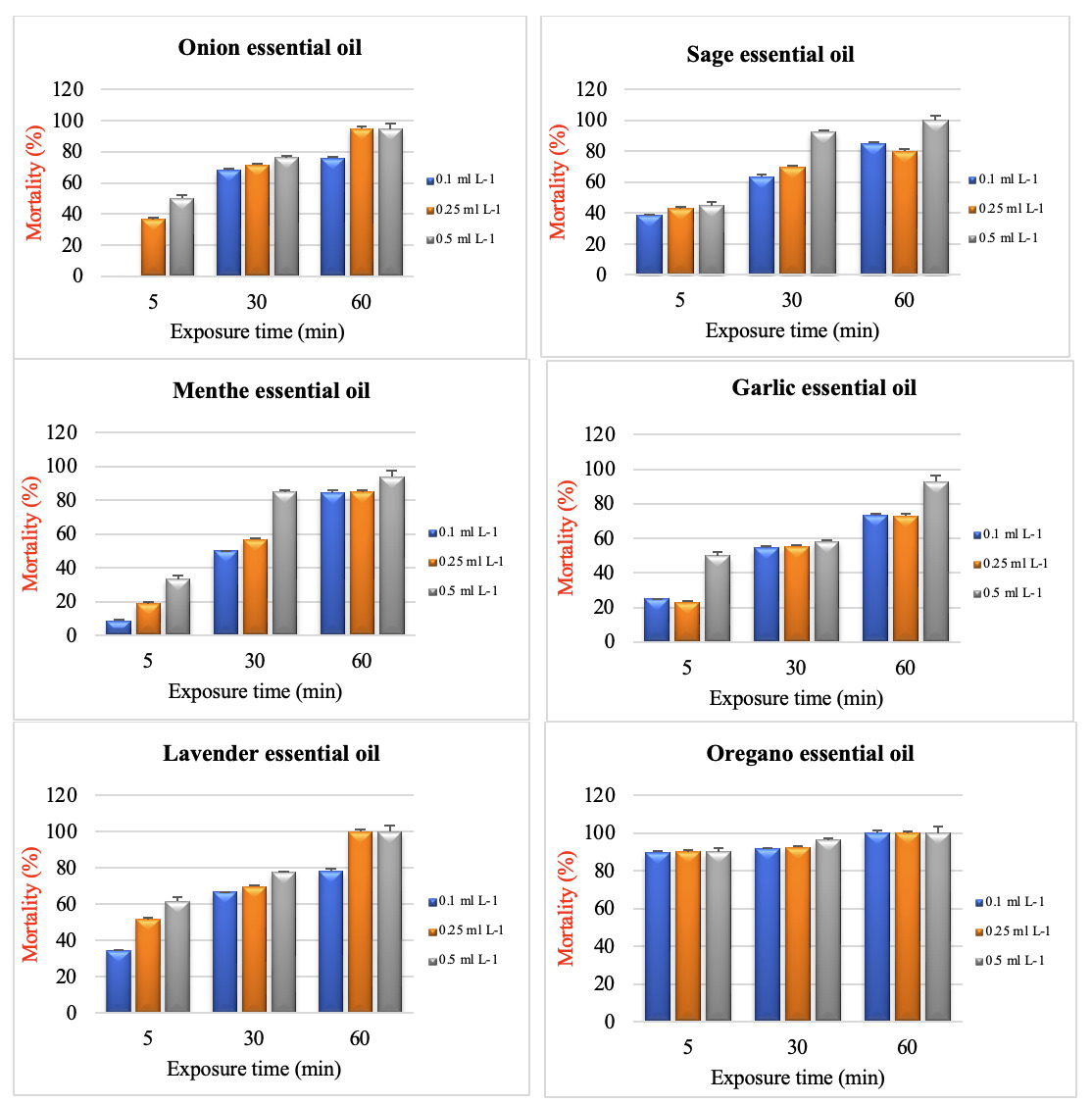
Antiparasitic efficacy correlated positively with the concentrations of the essential oils (p<0.05). Furthermore, at prolonged exposure, high concentrations of essential oils resulted in high mortalities on I. multifiliis trophonts (Figure 1). The in vitro antiparasitic efficacy of essential oils was shown in Figure 1, which showed that sage essential oil at 0.50 ml L-1, lavender essential oil at 0.25- and 0.50-ml L-1, oregano essential oil at 0.1, 0.25-, and 0.50-ml L-1 concentrations had a 100% antiparasitic effect against trophonts after 60 min of exposure (p<0.05). Onion, menthe, and garlic essential oil had lower mortality effects than other essential oils at concentrations of 0.1, 0.25-, and 0.50-ml L-1 trophonts ranging from 75-94% (onion), 84-94% (menthe), and 72-92% (garlic) at 60 min of exposure, respectively. Additionally, onion, sage, menthe, garlic, and lavender essential oils have effectiveness against trophonts at the concentration of 0.1, 0.25-, and 0.50-ml L-1 at 30 min, with the less effective same concentrations at 5 min. Oregano essential oil at concentrations of 0.1, 0.25-, and 0.50-ml L-1 exhibited the maximum antiparasitic efficacy on trophont mortality ranging from 92-96% at 30 min, whereas its essential oil has affected with a mortality rate of 89-90% at 5 min. The negative controls containing 0.1% DMSO caused no mortality of trophonts, while all trophonts were killed at formaldehyde (0.1 mL L-1) that was used as a positive control within 30 minutes.
Discussion
It is widely accepted that increased research activities have been demonstrated in using medicinal plants to treat parasitic diseases in fish. Most of these studies concentrated on the antiparasitic effects of bioactive compounds or plant extracts against I. multifiliis, such as berberine,10 curcumin,11 Cynatratoside-C,12 pentagalloylglucose,13 emodin,14 10-gingerol,15 the ethanol extracts of Chinese herbal medicines (Cynanchum atratum, Zingiber officinale, and Cynanchum paniculatum),16 aqueous extract of Capsicum frutescens,17 petroleum ether, chloroform, ethyl acetate, acetone, or methanol extract of Morus alba18 and alcoholic extract of Terminalia catappa L.19 Published reports mainly indicated that medicinal plants or their purified active compounds are effective on theront, encysted, and nonencysted tomonts life stages of I. multifiliis. However, in this study, antiprotozoal activity screening of essential oil proved effective in controlling the parasite’s infective stage (trophonts). In the life cycle of I. multifiliis, active trophonts on the host are covered with a thick layer of mucus. Therefore, these protective properties of trophonts make fighting and killing them more brutal than theront and nonencysted tomonts.12,20 The results showed that essential oils of sage at a concentration of 0.50 ml L-1, lavender at a concentration of 0.25- and 0.50-ml L-1, and oregano at a concentration of 0.1, 0.25-, and 0.50-ml L-1 can kill about 100% of I. multifiliis trophonts after 60 min of exposure. Furthermore, antiparasitic efficacy correlated positively with the high concentrations of the essential oils and prolonged exposure (p<0.05). There is still lack of literature on the efficacy of herbal essential oil in treating Ich infested fish. But further findings on the efficacy of herbal extracts in controlling I. multifiliis have been reported by Lesniak et al.,21 who opined that 3200 mg L-1 methanol extract of Eclipta alba and Arctium lappa destroyed 100% infective trohonts within 1 and 2 h of exposure, respectively. Macleaya cordata, Mucuna pruriens, and Carica papaya extract killed all trophonts at concentrations of 70, 150, and 200 mg L-1 within 4 and 6 h, respectively (Yao et al.22; Ekanem et al., 2011). Chelerythrine and chloroxylonine of the active components from Toddalia asiatica could be 100% effective against I. multifiliis at a concentration of 1.2 and 3.5 mg L−1.23 In contrast, essential oils from Varronia curassavica accessions the concentrations range of 10-200 mg L-1 provided 100% mortality of trophonts.24 In another study, Yao et al.25 prepared 2 fractions of Macleaya microcarpa. They determined that dihydrosanguinarine and dihydrochelerythrine were 100% effective at concentrations of 7.0 and 10.0 mg L-1 against I. multifiliis trophonts after 4 h of exposure, respectively. Chika et al.26 found that the infestation with I. multifiliis trophonts in the body and gill smear decreased by aqueous leaves extract of Moringa oleifera at concentrations of 1,500, 2,500, 3,500, and 4,5000 mg L-1 after 1 hour. In addition, similar to our study findings, they determined that the decrease in a number of trophonts present was concentration and time-dependent. Compared to the reported data in previous studies, in vitro study on the anti-Ich efficacy of sage, lavender, and oregano essential oils was better than other tested medicinal plants at lower concentrations.
Antiprotozoal activity of medicinal plants is attributed to their chemical composition, such as monoterpenes (1,8-cineole, alfa pinene, camphor, linalool, sabinene, and thujone), sesquiterpenes (e-nerolidol, germacrene B, and cadalene), isoprenoides, and terpenoids that include aldehydes, ethers, alcohols (carvacrol, thymol, eugenol, and borneol), and sulfur- or nitrogen-containing compounds (diallyl disulfide and indole).27 Considering the effect mechanisms of phytochemicals, some investigations have proven that these constituents indicated antimicrobial effects by disrupting the microbial membranes and influencing their permeability, inhibiting protein and DNA synthesis, interrupting virulence factors, etc.28 According to a previous antiparasitic activity study, Meschler et al.29 indicated that α form of thujone, monoterpene ketone, has been shown to display actions against helminths. Almohammed et al.30 obtained that Elettaria cardamomum L. essential oil and its main compound, 1,8-cineole exhibited a potent protoscolicidal effect against Echinococcus granulosus (cestod) larval stage by altering the permeability of plasma membrane. Marjanović et al.31 obtained the antinematodal effects of carvacrol and thymol on Caenorabditis elegans and Syphacia muris. Our study results demonstrated that the chemical contents of sage, lavender, and oregano, which have the highest antiprotozoal activity, have a high rate of α-thujone (46.80%), 1,8-cineole (32.65%), and carvacrol (73.00%), respectively. Therefore, we can attach the potent anti-Ich effect of tested essential oil to the presence of phytochemicals such as thujone, 1,8-cineole, carvacrol, etc.
In conclusion, our results showed that essential oil of sage, lavender, and oregano could kill I. multifiliis trophonts at 60 min, and could reduce the infectivity of trophonts at 5 and 30 min. However, further studies are required for antiparasitic activity and toxicity tests of these essential oils in the fish culture system.