Plant soot (Pulvis Fumi Carbonisatus) is the black soot attached to the bottom of the pot or chimney after plant burning. It has been used as traditional herbal medicine to stop bleeding, remove food retention, relieve inflammation, and exert detoxication.1 It could also be used as a pigment in food processing.2 Due to its pronounced pharmacological actions and high safety, plant soot was approved for use in feed additives by China in January 2020.1 It has been confirmed that plant soot could exert a growth-promoting effect by adsorbing intestinal mycotoxin, inhibiting bacterial infection, and promoting feed digestion in husbandry animals.1,3
Furthermore, plant soot has been widely applied to the disease treatment of terrestrial animals, such as enteritis, piglet diarrhea, bovine nosebleed, bovine urine blood, bovine gastroenteritis, bovine oral ulcer, sheep scabies, and rabbit mastitis. Recently, plant soot supplementation in diets was found to improve the growth performance of spiny eel (Mastacembelus aculeatus) and gibel carp (Carassius auratus gibelio) by ameliorating intestinal health.4,5 The aforesaid studies about the beneficial effects of plant soot on the intestinal health of limited aquatic animal species were conducted under laboratory conditions. Little information is available to confirm the beneficial effects on intestinal health under the practical culture condition.
As one of the most common fish species cultured worldwide, eel (Anguilla spp.) plays an important role in international trade. The American eel (Anguilla rostrata) is China’s main cultured eel species 6. This study evaluated the effects of plant soot supplementation on the intestinal health of American eels (Anguilla rostrata) farmed in the intensive system, which could provide a guideline for plant soot utilization effectively in aquaculture.
Materials and Methods
Experimental fish and design
American eels were cultured in Hexagon concrete tanks (Water surface area 320 m2, tank height 1.5 m, and water depth 0.9 m) in a commercial eel farm (Shunchang Shuntai Fishery Co., Ltd., Fujian Province, China) for 14 months. After grading, fish with similar body weight (238.10 ± 7.37 g) was distributed into nine tanks (5442 ± 57 kg/tank, about 22856 fish/tank), randomly divided into three treatment groups fed with diets containing different levels of plant soot supplementation. The three treatment groups, PS0 group, PS3 group, and PS5 group, were fed with the dietary plant soot supplementation levels at 0, 3 g/kg, and 5 g/kg, respectively. There were three replicates in each group. The trial lasted for 60 days.
Experimental design and fish-rearing management
The basal diet was a commercial diet provided by Fujian Tianma Science and Technology Group Co., Ltd., Fuzhou, China. The nutrient levels of the commercial diet were 47.43% crude protein, 6.35% crude fat, 13.30% ash, and 6.44% moisture. The plant soot was provided by Fujian Tanwawa Biotechnology Co., Ltd., Nanping, China, and it was manufactured by carbonization and activation of chippings of cedar and pine.
American eels were cultured in concrete tanks and fed daily (at 5:30 a.m. and 5:30 p.m.) until apparent satiation. The commercial diet was mixed with a ratio of 1: 1.2 in water volume to form a dough shape. Then it was placed on a feeding table to feed the eels. The following water quality parameters were maintained during the acclimation period: temperature 23 -27 °C, pH 6.9 - 7.5, dissolved oxygen 7.5 - 9.2 mg/L, total ammonia nitrogen <0.10 mg/L, and nitrite nitrogen level <0.01 mg/L. Water exchange of approximately 100 m3/tank was done twice daily (6:30 a.m. and 6:30 p.m.).
Sample collection
At the end of the feeding trial, eleven fish were randomly selected from each tank and anesthetized with 2 g/L eugenol.
The blood samples of five fish per tank were collected from the caudal vein. After holding at 4°C for 30 min, the blood samples were centrifuged at 3500 rpm for 10 min at 4 °C, and the supernatants were collected. The supernatant samples from each tank were pooled and stored at -80 °C before serum parameter analysis. After blood sampling, the foregut samples were dissected and rinsed with normal saline, then frozen with liquid nitrogen in sterilization freeze tubes to analyze intestinal microbial diversity.
The rest of the anesthetized eels, six fish per tank, were kept in an ice bath for midgut samples. The three midgut samples were immersed in Bouin’s solution for morphological observation. Another three midgut samples per tank were taken, fixed, and stored at 4 °C for scanning electron microscopy examination.
Analytical procedures
According to the method of Wang et al.,7 the D-lactate level and Diamine oxidase (DAO) activity in the serum were measured by using commercial kits (Shanghai Jianglai Biotechnology Co., LTD, China) with enzyme-linked immunosorbent assay methods.
The methods for preparing intestinal tissue sections and measuring the intestinal villus height and muscular thickness were the same as the study of Lu et al.8 The sections were stained with hematoxylin and eosin (H & E) and examined with a positive fluorescence microscope (BX80-JPA, Olympus, Tokyo, Japan) to take representative photomicrographs. Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA) was used for morphometric analysis.
Samples of intestines for scanning electron microscopy were treated as described in the study of Lu et al.8 Midgut samples were fixed in glutaraldehyde for at least 24 hours before being cleaned with phosphoric acid buffer (0.1M) three times and then dehydrated with a graded ethanol series. The treated samples were dried in a critical point dryer (K850, Quorum, UK) and then gold coated in an ion sputtering instrument (MC1000, Hitachi, Tokyo, Japan) before being observed and photographed on a scanning electron microscope (SU8100, Hitachi, Tokyo, Japan).
Five intestine samples from each tank were used for microbiota analysis by the 16S rDNA high-throughput sequencing method. The high-throughput sequencing of intestinal microbiota was performed by the Illumina Miseq PE300 platform (Beijing Allwegene Tech. Co., Ltd., Beijing, China). The extraction and quality detection of intestinal total DNA, the design of primers for PCR amplification of bacterial 16S rDNA V3-V4 region, and data analysis were the same as the description by Shi et al.9
Statistical analysis
The values of D-lactate and diamine oxidase in the serum and villi length and muscular layer thickness of the intestine were presented as means ± SD. Statistical analysis was performed with SPSS 22.0 statistical software (SPSS, Chicago, IL, USA). Duncan’s test was used for multiple comparisons if significant differences existed. P <0.05 indicated a statistically significant difference. The alpha diversity indices were analyzed using QIIME (v1.8.0) software and LEfSe analysis was performed using R-Statistical software v3.6.0 (R-Statistical Corp., Vienna, Austria). The Linear Discriminant Analysis (LDA) score screening value was set to 3.5, P<0.05.
Results
Parameters in serum related to the intestinal barrier function
The effects of dietary plant soot supplementation on D-lactate level and DAO activity in the serum of American eels are shown in Table 1. The D-lactate level and DAO activity in the serum of the PS5 group were significantly lower than those of the PS0 and PS3 groups (P <0.05), and there were no significant differences between those two parameters between the PS0 group and PS3 group (P>0.05).
Intestinal Morphology
The results of dietary plant soot supplementation on the intestinal morphology of American eels are shown in Figure 1, Table 2, and Figure 2, respectively. Compared with the PS0 group, the villi length of the intestine in both PS3 group and PS5 group significantly increased (P<0.05). The muscular thickness of the intestine of the PS5 group was significantly higher than the PS0 group (P<0.05). The microvillus density of the intestine of the American eel was increased obviously in the PS5 group.
Intestinal microbiota
The alpha diversity indices of intestinal microbiota are shown in Figure 3. There was no significant difference in the alpha diversity indices of the intestinal microbiota among the PS0, PS3, and PS5 groups (P>0.05), and it indicated that dietary plant soot supplementation did not affect the abundance and diversity of bacteria in the intestine of farmed American eels.
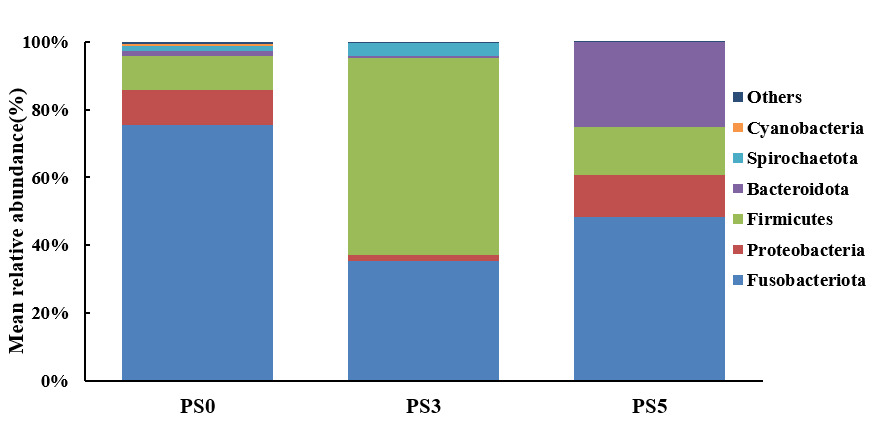
The intestinal microbiota composition at the phylum level is shown in Figure 4. Compared with the PS0 group, the relative abundances of Fusobacteriota in the PS3 and PS5 groups were decreased, while the relative abundance of Firmicutes was increased in the PS3 group. The relative abundance of Bacteroidota in PS5 was higher than the other two groups.
The LEfSe analysis of intestinal microbiota was shown in Figure 5. The relative abundances of Shewanella and Aeromonas in the PS0 group were significantly higher than those in the other groups (P<0.05). While the relative abundances of Geobacillus, Bacillus, and Candidatus Arthromitus in the PS3 group were significantly higher than those in the PS0 group and PS5 group (P<0.05), the relative abundances of Photobacterium and Plesiomonas in PS5 group were significantly higher than those in the PS0 and PS5 groups (P<0.05).
Discussion
At present, little information is available about the parameter of intestinal health affected by dietary plant soot supplementation. Only the activities of digestive enzymes in the gut of spiny eel and gibel carp were reported.4,5 D-lactate level and DAO activity in serum are relevant to the integrity and permeability of the intestine, and their decreased values mirror the improvement of mucosal barrier function.10 The decrease of D-lactate level and DAO activity in the present study indicated that dietary plant soot supplementation might improve mucosal barrier function in American eels. The villi length, muscular thickness, and microvillus density of intestinal mucosa can directly reflect fish’s digestive and absorption capacity.8,11,12 In the present study, the morphological improvement, including villi length, muscular thickness, and microvillus density in the intestine of American eels, might increase the area of the mucosal surface. Improving mucosal surface area could enhance the ability of intestinal digestion and absorption.7,11
The alpha diversity indices of bacteria are usually used to evaluate the species abundance and richness of the intestine microbiota.13 There was no significant difference in the alpha diversity indices, indicating that dietary plant soot supplementation could not affect the abundance and diversity of bacteria in farmed American eels’ intestines.
It has been reported that the decreased relative abundance of Fusobacterium might reduce the incidence of intestinal disease.14 Also, the Firmicutes might be beneficial to stimulate the absorption and metabolism of fatty acids in the fish gut.15 The higher relative abundance of Bacteroidota might benefit the mucosal immune system in the intestine of American eels.7 In the present study, the increased relative abundances of Fusobacteriota and Bacteroidota in PS groups were accompanied by the decreased relative abundance of Firmicutes. These results suggested that the intestinal microbiota composition of the farmed American eel might be influenced beneficially by dietary plant soot supplementation.
Shewanella and Aeromonas were widely reported as potential opportunistic pathogens in the fish intestine.16 The higher relative abundance of those bacteria of the PS0 group in the present study indicated that the American eel without plant soot supplementation might suffer more attacks of pathogens in the intestine.
Geobacillus was reported to provide heat-stable proteins with unique bioenergy production capacity and a variety of heat-resistant enzymes for degrading cellulose and starch, and it might act as a probiotic to improve the growth performance, antioxidant and immune response of some fish species.17 As a dietary source of probiotics, Bacillus spp. can enhance aquatic animals’ growth and immune function.18 Candidatus Arthromitus is a natural member of the gut microbiota in some fish species. It could modulate the host immune response and intestinal epithelial cell function and reduce the risk of pathogenic infection.19 The increased relative abundances of those three bacteria in the intestine of American eels were shown in the PS3 group, which suggested that dietary plant soot supplementation could exert beneficial effects on the intestinal health status by the increase of some probiotic bacteria.
Photobacterium and Plesiomonas were considered potential opportunistic pathogens in the intestine of some fish species.9,20,21 Although the relative abundances of Photobacterium and Plesiomonas were higher in the intestine of American eels in the PS5 group, no symptom of intestine health problems was observed in the sampling process and cultivation. The intestinal health status of the American eel in the PS5 group was best in the sampling process and cultivation among the three groups, which might also be reflected by the changes in parameters related to intestinal barrier function and intestinal morphology in the present study. Similarly, the relative abundances of Plesiomonas and Photobacterium in the intestines of fast-growing European eels were significantly higher than the slow-growing ones.9 The Plesiomonas abundance in the healthy gut of catfish was significantly higher than the fish with intestinal sepsis.21 Those bacteria may not be pathogenic to American eels. Their function in the intestine of American eels should be studied further. Up to now, there is no other report about the effects of plant soot on the intestine microbiota of aquatic animals. The mechanism of plant soot on the composition and relative abundances of intestine microbiota in American eels should be investigated in future studies.
In conclusion, dietary plant soot supplementation might regulate the intestinal health of the farmed American eels by strengthening the intestinal barrier function, improving the intestinal morphology, and beneficially modulating intestinal microbiota with the higher relative abundances of certain probiotics and the lower relative abundance of some pathogenic bacteria. Dietary 5 g/kg plant soot supplementation could exert certain beneficial effects on the intestinal health of farmed American eels.
Acknowledgments
This work was supported by the Regional Development Project for Science and Technology Plan Program of Fujian Province under Grant No.2022N3002 and the earmarked fund for the China Agriculture Research System of MOF and MARA under Grant No. CARS-46.
Author contributions
Formal Analysis: Yi-yao Yu (Equal), Qian Yin (Equal), Ming-liang Zhang (Supporting). Investigation: Yi-yao Yu (Equal), Qian Yin (Equal), Ming-liang Zhang (Supporting). Writing – original draft: Yi-yao Yu (Equal), Qian Yin (Equal). Writing – review & editing: Feng Xi (Equal), Shao-wei Zhai (Equal). Resources: Feng Xi (Equal). Conceptualization: Shao-wei Zhai (Lead). Methodology: Shao-wei Zhai (Lead). Funding acquisition: Shao-wei Zhai (Lead). Supervision: Shao-wei Zhai (Lead).

__ps3_group_(b)__and_ps5_gr.png)
__ps3_group_(b)_.png)



__ps3_group_(b)__and_ps5_gr.png)
__ps3_group_(b)_.png)