Introduction
The In-Pond Raceway System (IPRS) utilizes a series of tanks occupying 2% to 5% of the pond area,1–3 equipped with aerators and pollution collection devices, as the cultivation area, while the remaining 95% to 98% of the water surface is used as the purification area.4,5 This enables the recycling of water used in the cultivation process throughout the cultivation cycle. Emodin plays a crucial role as an immunostimulant in aquaculture. Previous studies have shown that it can increase the number of fish immune-related phagocytes and the activity of related enzymes, activate the fish immune system, and significantly enhance the body’s nonspecific immune function. Emodin has been demonstrated to enhance the thermo-tolerance of Megalobrama amblycephala and promote growth performance, as well as increase the innate immune response and resistance to pathogenic bacterial infections in Carassius auratus,6 Macrobrachium rosenbergii,7 and M. amblycephala.8 Using comparative proteomics research techniques, it has been demonstrated that emodin can enhance the antioxidant capacity of M. amblycephala.9 Furthermore, it has been found that emodin can improve the oxidative stress resistance of peripheral leukocytes in M. amblycephala by activating the Nrf2-Keap1 signaling pathway.10 Therefore, studying the effects of emodin extract on M. amblycephala in IPRS has important implications.
Protein, as a fundamental and critical component of the body’s life activities, plays a vital role in the growth and development of organisms. Protein deposition in fish is a prerequisite for their growth and weight gain, yet it is also a relatively expensive nutrient in feed ingredients. Protein possesses multiple biological functions, such as supporting growth (increasing body protein), serving as a structural material, acting as a carrier for transportation, producing antibodies for immunity, catalyzing enzymes, regulating hormones, providing collagen, and serving as an energy source.11 However, excessive addition of protein in feed relative to energy can lead to protein being consumed as an energy source, resulting in increased feed costs and increased discharge of nitrogen-containing substances that pollute the aquatic environment.12 Therefore, research on the effects of protein levels on aquatic animals is of great significance for developing nutritionally balanced, economical, and efficient fish feed.
This research investigates whether emodin has a protective effect on M. amblycephala in IPRS. In addition, the effects of different protein levels on M. amblycephala were investigated. The study focused on the growth, physiological health, and immune performance of M. amblycephala, providing basic data and a theoretical basis for the development of formulated feed for M. amblycephala in IPRS, and promoting the production and development of M. amblycephala in aquaculture.
Materials and Methods
Ethics statement
All experiments were performed following the guidelines on the care and use of animals for scientific purposes set by the Institutional Animal Care and Use Committee (IACUC) of the Chinese Academy of Fishery Science (CAFS). This study was also specifically approved by the Committee on the Ethics of Animal Experiments of Freshwater Fisheries Research Center at the Chinese Academy of Fishery Science. All efforts were made to minimize animal suffering.
Experimental fish and feeding trial
The Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Yangzhong, China) provided the culture pond and fish. Before the feeding trial, the fish underwent 2 weeks of acclimation to the experimental diet and conditions. After acclimation, fish with an average body weight of 12g were randomly divided into 3 groups, with three replicates for each group. Each pond was stocked with 20,000 fish. Commercial feed (Table 1) provided by Dabeinong Feedstuffs Co., Ltd. (Huaian, China). Based on a previous study performed in our laboratory,13 the dietary protein requirement of Wuchang bream is 32-33%. Therefore, to gain obvious physiological and immunity effects of emodin and different protein levels on juvenile blunt snout bream, protein levels in this experiment were designed to 29% and 32%, respectively. and mainly composed of fish meal, soybean meal, and vegetable meal.
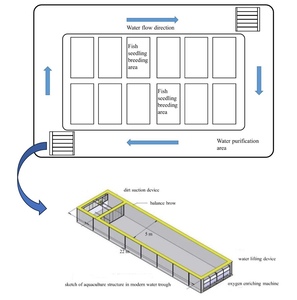
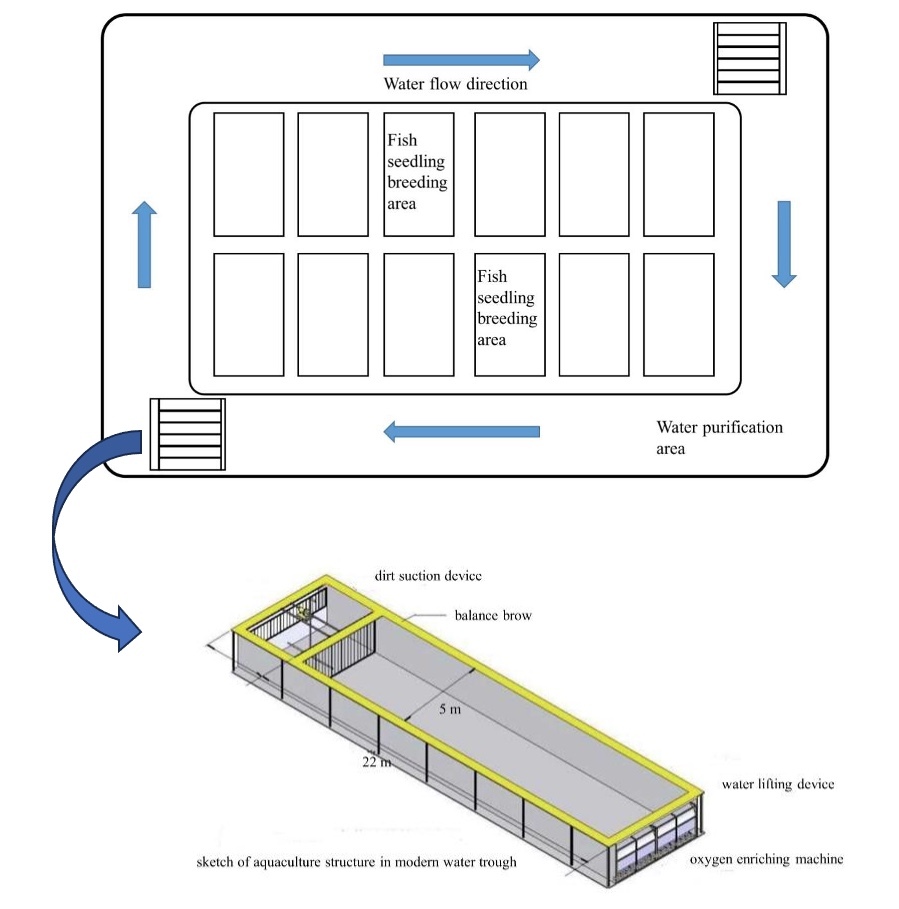
The fishery IPRS was installed in rectangle troughs (22 × 5 × 2.5 m) in a circular pond with an average water depth of 2.2 m. The system uses the principle of recirculating aquaculture, including plant purification and filter-feeding fish (Figure 1). An inflatable aerator was used to promote water flow and ensure that the culturing area was well oxygenated in each raceway pond. The feces were centralized and removed by the sewage facilities. Recirculating system monitors (Yantai Dongrun Co. Ltd., China) were installed to monitor both DO and temperature in the outflow water of each raceway. The average flow rate of the water in all raceways was 0.08 m/s (0.88 m3/s). The temperature was maintained between 26 and 31 °C throughout the experimental period. Other water quality parameters were maintained at 7.4–7.8 pH, dissolved oxygen ≥5 mg L−1, and ammonia nitrogen < 0.1 mg L−1.
Sampling
At an interval of 30 days, a random sample of 100 fish was taken from each pond to measure their weight and length. The fish were then returned to their respective ponds. To perform the activity of blood parameters, 9 individuals from each experimental group (3 fish/trough, 3 troughs/group) were euthanized on day 210. Fish were carefully taken from each raceway trough and transferred to a new tank containing diluted MS-222 at a concentration of 100 mg L−1. The blood samples were collected by caudal vein puncture, transferred to heparin tubes, and kept on ice until centrifugation. After collection, 20μL of blood in each fish was used for hemocyte analyses. The remaining blood was centrifuged at 3000 ×g at 4 °C for 10 min to collect plasma, after which the plasma was stored at −80 °C and used for immunological assays.
Measurement of blood parameters
The levels of triglyceride (TG), albumin (ALB), alkaline phosphatase (ALP), glucose (GLU), aspartate transaminase (AST), and alanine aminotransferase (ALT) in the plasma were tested with a Mindray BS-400 automatic biochemical analyzer (Mindray Medical International Ltd., Shenzhen, China), as described in our previous study.14 The WBC count, red blood cell (RBC) count, hemoglobin content, and HCT were determined using an auto-hematology analyzer (BC- 5300Vet; Mindray, Shenzhen, PR China) and test kits from Shenzhen Mindray Medical International Co. Ltd., PR China, according to a previously described method.15
Antioxidant-related parameters assay
The levels of superoxide dismutase (SOD), anti-superoxide anion free radical (ASAFR), malondialdehyde (MDA), nitric oxide synthase (iNOS), nitric oxide (NO), catalase (CAT), glutathione peroxidase (GSH-Px) activity were measured using the reagent kits and methods provided by Nanjing Institute of Biological Engineering, according to a previously described method.15
Statistical analysis
All data were presented as mean ± SEM (standard error of the mean). Data were transformed, if necessary, after evaluating assumptions of normality, equality of variances, and outliers and subjected to one–way analysis of variance (ANOVA) using the software SPSS 22.0 (International Business Machines Corporation, Armonk, New York, USA) for Windows. Tukey’s multiple-range test evaluated significant differences in the means between dietary treatments. Mean differences were considered significant at a P value equal to or less than 0.05.
Results
Growth performance
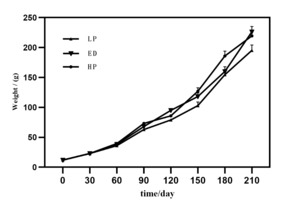
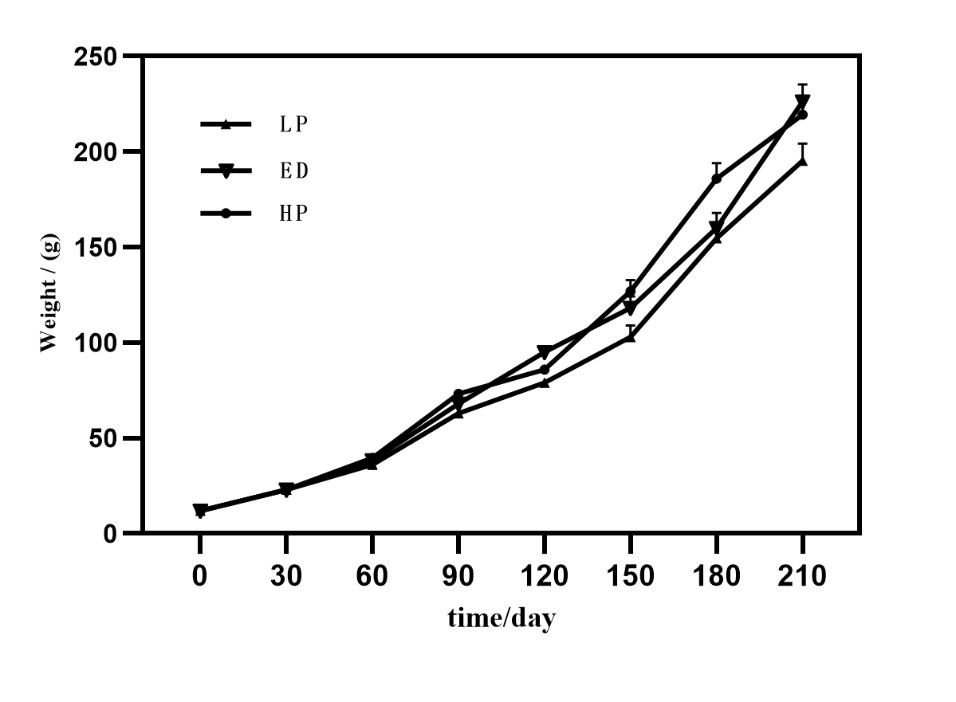
There were no disease outbreaks throughout the experimental period, and the survival rate was exceptionally high across all treatment groups, exceeding 99%. As Figure 2 demonstrates, the body weight of M. amblycephala experienced exponential growth over time. By day 210 of the rearing period, the body weight of the ED and HP groups surpassed that of the LP groups, with a statistically significant difference (P < 0.05). By day 210 of the rearing period, the final body weights were 195.37±19.1g, 226.3±17.2g, and 219.5±15.3g in the LP, ED, and HP groups, respectively. The levels of emodin and different protein levels did not significantly impact the hepatosomatic index (HSI) or viscerosomatic index (VSI) of M. amblycephala. The ED and HD groups showed significant improvement in weight gain rate and reduced feed conversion ratio compared to the LP group (P < 0.05) (Table 2).
Hematological parameters
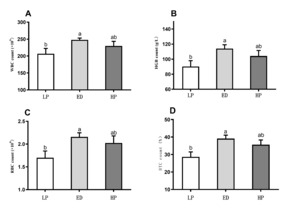
Figure 3 presents the difference analysis results of hematological indicators (WBC, RBC, HGB, and HCT contents). After 210 days of breeding, the contents of WBC, RBC, HGB, and HCT in the ED group were significantly higher than those in the LP group (P < 0.05). However, no significant difference was observed between the HP and LP groups.
The changes in serum biochemical parameters
After 210 days of breeding, the ED group showed a significant increase in the serum levels of TP, ALB, and GLU of M. amblycephala compared to the LP group (P < 0.05. Figure 4). Additionally, the levels of TG and TC in ED group were significantly decreased than that of control group (P < 0.05. Figure 4). Besides, the HP group exhibited a significant increase in the serum level of CREA compared to the LP group (P < 0.05. Figure 4), and the levels of TG were significantly decreased compared to the LP group.
The changes in antioxidant parameters.
After 210 days, the serum GPx, SOD, ASAFR and NO enzyme activities in the ED group exhibited a significant increase compared to the LP group (P < 0.05. Figure 5). Additionally, the serum MDA content in the ED group were significantly decreased compared to the LP group (P < 0.05. Figure 5). The HP group, showed a significant increase in the serum GPx, SOD, ASAFR and NO enzyme activities compared to the LP group (P < 0.05. Figure 5).
Discussion
The emodin contains abundant bioactive substances such as alkaloids, polysaccharides, saponins, and anthraquinones that are closely related to the immune function of living organisms. It has been widely used in aquaculture.16 In this study, compared with the control group, adding emodin (500mg/kg) to the basal diet of M. amblycephala can significantly promote fish growth and reduce FCR. These results are consistent with previous research findings.7
The liver is the primary metabolic organ in fish, and liver morphology indicators such as HSI, and VIS can reflect liver function status in cases of malnutrition or overfeeding in fish. A higher VSI increases the likelihood of liver diseases such as hepatomegaly and fatty liver in fish.17 This study showed that feed protein levels did not influence feeding quantity, HSI, and VSI. This is similar to the previous research results on Scortum Barcoo.18
The blood indices are commonly used as physiological, pathological, and toxicological markers, which are widely applied in the assessment of the health and nutritional status of fish.19 Emodin supplementation in the feed led to a consistent elevation of various blood cell indices (WBC, RBC, HGB, and HCT), corroborating previous research on the blunt-snout bream.20 This may be attributed to the ability of emodin to enhance fish metabolism, strengthen the fish immune system, and increase oxygen consumption due to the increased metabolism. However, the different protein levels did not significantly affect the hematological parameters in this experiment. This is similar to the previous research results on M. amblycephala.13
AST and ALT are the two most important transaminases in fish. In this experiment, there was no significant difference in AST and ALT levels between the ED group and the HP group, and the liver did not exhibit any phenomena such as enlargement or atrophy, indicating that the fish liver’s metabolic function was normal. TP and ALB in the serum can indirectly reflect the body’s non-specific immune ability. In this experiment, the ED group significantly increased the plasma TP and ALB content, similar to previous research on Procambarus clarkia21 and Acipenser schrenckii.22 In addition, the protein level in the feed had no significant effect on the TP and ALB in the serum of blunt snout bream, indicating that they may adjust protein homeostasis to adapt to the aquaculture environment. The normality of blood glucose concentration (GLU) plays a crucial role in maintaining the normal life activities of fish. In this experiment, the ED group’s GLU significantly increased compared to the control group, indicating that emodin may promote fish metabolism and strengthen the body’s energy utilization. A similar result has been reported in Cyprinus carpio var. Jian.23 The variation in the levels of cholesterol and triglycerides in the plasma reflects, to a certain extent, the state of lipid metabolism in the liver.24 In this study, the levels of triglycerides and cholesterol in the ED group were significantly elevated compared to the control group. Previously, the addition of anthraquinone extract to the diet of Procambarus clarkii could significantly increase the levels of cholesterol and triglycerides in the lymphatic fluid, which also suggests that emodin can enhance metabolism and meet the energy demands under stress conditions.7
The iNOS enzyme possesses the capability to scavenge oxygen-free radicals. In this experiment, the administration of emodin significantly increased the serum iNOS concentration, which is consistent with the findings observed in experiments conducted on grass carp25 and Cyprinus carpio L.26 During normal metabolic processes in the body, there is a dynamic balance between the production and metabolism of reactive oxygen species (ROS), which include superoxide anion (O2-), hydrogen peroxide (H2O2), ion (•OH), and others. Excessive free radicals can lead to lipid peroxidation, and O2- can be produced during immune activities such as phagocytosis and secretion of cytokines. The antioxidant enzyme system composed of superoxide dismutase (SOD), anti-superoxide anion factors (ASAFR), and catalase (CAT) can clear excess free radicals and play an important role in clearing oxidative stress, enhancing the defense capacity of phagocytic cells, and improving the body’s immune function. The results of this experiment demonstrate that the HD group exhibited a significant increase in the GPX and SOD levels, indicating that a high-protein diet can enhance the antioxidant capacity of the blunt snout bream. This is similar to the findings of previous studies on Carassius auratus gibelio,27 and Carassius auratus Gibelio.28 In this experiment, the addition of emodin significantly increased the levels of antioxidant enzymes such as SOD, CAT, and GPx compared to the control group. MDA is a product of lipid peroxidation, generated by the action of free radicals on lipids, and has strong biotoxicity that can severely disrupt cellular structure and function. The MDA content of the group with emodin addition was significantly lower than that of the control group, consistent with previous research. This experiment suggests that adding emodin to the diet within a certain range and increasing protein levels can enhance the body’s antioxidant capacity.
Conclusions
To conclude, this study provides evidence that incorporating emodin (500mg/kg) into the basal diet of M. amblycephalain can effectively enhance fish growth and reduce FCR. Emodin achieves this by bolstering fish metabolism, strengthening the immune system, and producing a positive trend in WBC, RBC, HGB, and HCT counts. Additionally, it increases TP, GLU, and ALB levels in the serum’s biochemical parameters. Furthermore, appropriate protein levels can increase the content of antioxidant enzymes, enhance immunity, and promote the growth of M. amblycephala.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32172990); the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD59). The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
Authors’ Contribution per CRediT
Conceptualization: Zhenfei Yang (Equal), Haiyue Cao (Equal), Bo Liu (Lead). Writing – original draft: Zhenfei Yang (Equal), Haiyue Cao (Equal), Liangwei Xiong (Equal). Writing – review & editing: Zhenfei Yang (Equal), Haiyue Cao (Equal), Wei Li (Equal), Fugang Qi (Equal). Methodology: Pao Xu (Equal), Jianguo Wang (Equal). Funding acquisition: Jianguo Wang (Lead), Quan Wang (Lead). Formal Analysis: Apeng Lin (Lead). Investigation: Apeng Lin (Lead). Resources: Wenxiang Yao (Lead). Supervision: Xiaofeng Tang (Lead).