Introduction
As a new group of feed additives in aquaculture, natural phytobiotics (antibacterial additives of plant origins) are widely used in diets to avoid antibiotic resistance. Those phytobiotics exhibit many effects, particularly antimicrobial, gut microbiota manipulation, intestinal barrier improvement, antioxidant, anti-inflammatory, nutrient absorption, and immune modulation, which are beneficial for improving growth performance and health of aquatic animals.1–3 Among phytobiotics resources, Macleaya cordata, was lately received greater attention in the field of animal production. This medicinal plant is a perennial and traditional herb with a long history, primarily distributed in China, North America, and Europe. Macleaya cordata extract (MCE) contains many vital alkaloids, primarily sanguinarine and chelerythrine.4–6 Those alkaloids have broad antimicrobial activity as well as anti-inflammatory properties. Due to its pronouncedly pharmacological effects and high safety, MCE has been approved as a feed additive in the EU and China and standardized to 1.5% w/w sanguinarine.3,4 The beneficial effects of MCE have been extensively demonstrated in promoting the growth performance and health status of livestock animals and poultry.4 It has been confirmed that the beneficial modulation of gastrointestinal microflora might cause the positive effects of MCE on the gut health of animals. A similar phenomenon was also reported in aquatic animals, including red tilapia (Oreochromis niloticus),7 Koi carp (Cryprinus carpio),1,8 grass carp (Ctenopharyngodon idellus),9 and American eel (Anguilla rostrata).3,10 It is necessary to investigate the beneficial effects of MCE on the intestinal microflora of other fish species for further application.
Eel (Anguillia spp.) is an economically important fish species for freshwater aquaculture in the world. The main cultured eel species are the Japanese eel (Anguilla japonica) and the American eel in China. With the natural stocks of European eel declining sharply, it is listed in Appendix II of the IUCN as a Critically Endangered Species. However, there are still limited quantities of Anguilla anguilla glass eels imported into China yearly.11 Although dietary supplementation with MCE appears to exert beneficial effects on the intestinal microflora of American eels in previous studies,3,10 no research is available about the application of MCE in the diet of the European eel. It was proved that there were some differences in the intestinal microflora community between European eel and American eel.12 Therefore, this trial evaluated the effects of dietary MCE supplementation on the intestinal microflora of European eels, which might provide some references for using MCE in European eel culture.
Materials and Methods
Experimental fish and design
The experimental fish were European eels intensively cultured in Hexagon cement tanks (Water surface area 330 m2 with 1.2 m height and 1.0 m water depth) in a commercial eel farm (Huaqiang Eel Farm in Fujian Province, China). The individual growth rates are substantially different during eel culture, and grading eels about every two months is necessary to reach a high overall growth performance. After grading eels, six cement tanks with similar body size and fish weight (about 167 g/fish and 3620 kg/tank) were randomly divided into two treatment groups (control and MCE groups) with three tanks per group. The control group was fed a commercial diet, and the MCE group was fed the same commercial diet supplemented with 100 mg/kg MCE. This trial lasted for six weeks.
Experimental diet and fish management
The commercial diet (provided by Fujian Tianma Science and Technology Group Co., Ltd., Fuzhou, China) contained 48.12% crude protein, 4.74% lipids, and 16.69% ash. MCE (An orange powder product containing 1.5% sanguinarine and 0.75% chelerythrine) was manufactured by Hunan Micolta Bioresource Co., Ltd., Changsha, China. The batch number of the MCE product was 21-03-291. Fish were fed twice daily (5:00 and 17:00) at 2% of body weight (BW). The uneaten feed was taken out with a net 30 min after feeding and dried. The consumption of diet was recorded daily. The water temperature was kept at 25-30°C. Water quality variables were pH 7-8.5, dissolved oxygen 6-12 mg/L, total ammonia nitrogen 0.4-0.9 mg/L, and nitrite nitrogen levels <1.0 mg/L.
Sample collection and analysis
On the final day of the trial, eight fish from each group were sampled randomly after 24 h fasting. Then the fish were transported on ice, anesthetized with 200 mg/L MS-222 for 10-15 min, and rinsed with 75% ethanol before dissection with sterile scissors. The intestine samples without contents were separated from the abdominal cavity and flushed with PBS buffer to remove feces for high throughput sequencing analysis. Total bacterial DNA was extracted, and bacterial 16S rDNA sequences spanning the variable regions V3–V4 were amplified according to the previously described method.13 The Illumina Miseq PE300 platform was used for high throughput sequencing analysis with Beijing Allwegene Technology Co., Ltd. (Beijing, China). The raw data sequences were spliced and filtered to obtain high-quality sequences for further analysis.
Statistical analyses
Those data from each treatment group were subjected to a one-way analysis of variance (ANOVA) with SPSS 22.0 statistical software (SPSS, Chicago, IL, USA). When overall differences were significant (P<0.05), Duncan’s multiple range test was used to compare the mean values among different treatment groups. Linear discriminant analysis (LDA) effect size (LEfSe) was performed to present biomarkers of gut microbial communities at genus level between the control group and MCE group discriminated by nonparametric factorial Kruskal–Wallis test and pairwise Wilcoxon test with the P value <0.05. The LDA score threshold was 3.
Results
The alpha diversity indexes of the intestinal microbiota
The alpha diversity indexes of the intestinal microbiota of European eels between the control and MCE group are shown in Figure 1. The Chao1 index,observed-species index, PD-whole-tree index, and Shannon index in the MCE group were higher than those in the control group.
The Beta diversity of the intestinal microbiota
As shown in Figure 2, PC1 was 33.59 %, and PC2 was 14.89 %. There was no intersection between the two groups, and the samples in the group were clustered closely. Those results indicated that the samples from different groups were separated, and there were apparent differences of intestinal microbiota between the control group and the MCE group.
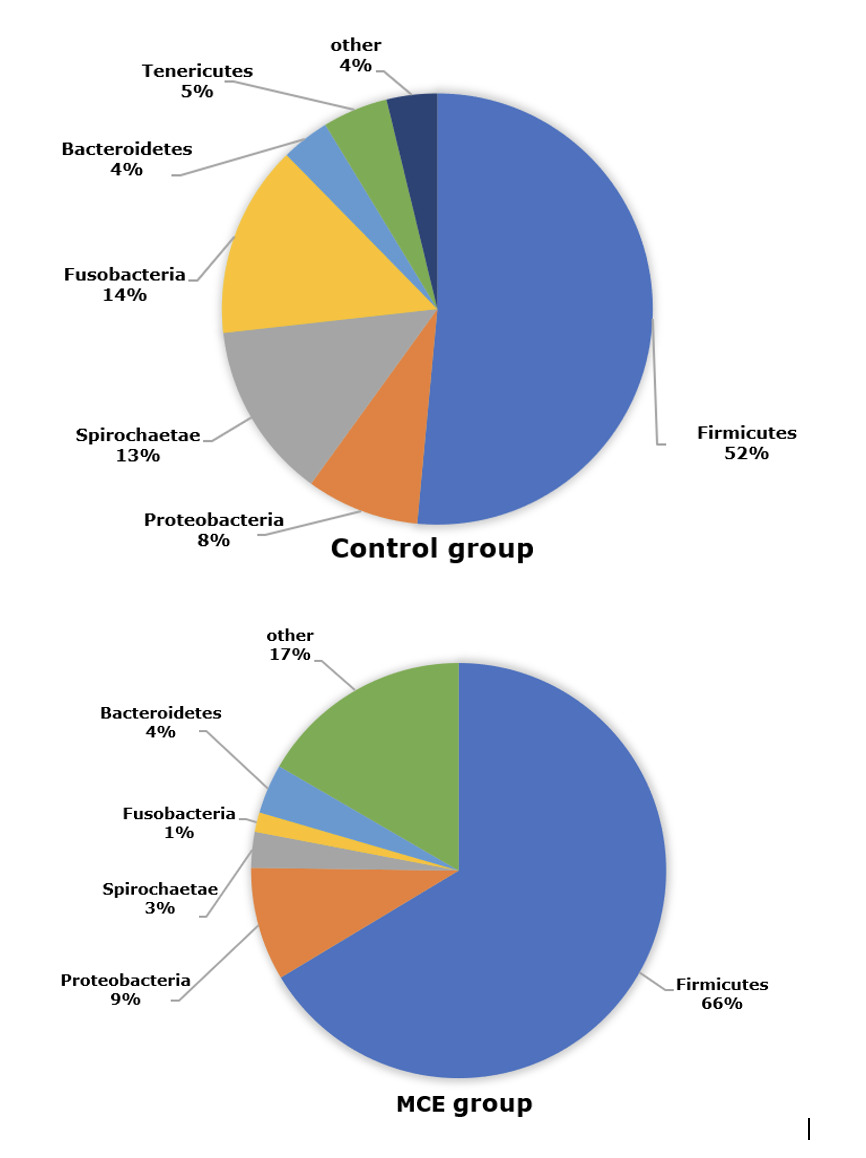
The composition of intestinal microbiota at the phylum level
The composition of intestinal microbiota at the phylum level of European eels in control and MCE groups is shown in Figure 3. The top three predominant phylum of European eels in the control group were Firmicutes, Proteobacteria, and Spirochaetae. While in the MCE group, there was an increasing trend of relative abundance of Firmicutes and Proteobacteria, and a decreasing trend of the relative abundances of Spirochaetae and Fusobacteria.
LEfSe analysis of intestinal microbiota at the genus level
Based on the LEfSe analysis, the differential bacteria of the intestinal microbiota at the genus level of European eels are shown in Figure 4. The relative abundances of Streptococcus and Legionella were significantly higher in the control group (P<0.05). The relative abundances of Bacillus, Anaerobacillus, and Sphingomonas were significantly higher in the MCE group (P<0.05).
Discussion
The intestinal microbiota plays an essential role in fish health by providing vital nutrients and stimulating the innate immune response.14 The abundance and diversity of the microbial community could be revealed by alpha diversity analysis. The Chao 1 and observed species were usually employed to represent the richness of bacteria species, and the higher values of the PD-whole-tree index and Shannon index indicated that there might be a greater diversity of the bacteria species.15 In the present study, the MCE group showed higher values of Chao 1, observed-species, PD-whole-tree, and Shannon compared with the control group, which indicated that dietary MCE supplementation might improve the species abundance and richness of the intestinal microbiota of European eel. Similar results were reported in the study of MCE supplementation in the diet of koi carp.1 In addition, the beta diversity of the intestinal microbiota by PLS-DA analysis showed a significant difference between the control and MCE groups, suggesting that MCE supplementation could change the intestinal microbiota composition of European eels.
In the present trial, Firmicutes, Proteobacteria, and Spirochaetae were the dominant bacteria in the intestine of European eel. Similar results were observed in a previous study of European eel.13 The relative abundances of both Firmicutes and Proteobacteria were increased in the MCE group, which might be beneficial to gut health according to the changes of those bacteria in the intestine of the European eel from the fast-growth group in comparison with the stunted growth group.13 Firmicutes and Proteobacteria were also considered to produce protease, the Firmicutes might assist in the nutritional processes of complex and undigested polysaccharides,16 stimulate the absorption and metabolism of fatty acids in the fish gut, and promote host energy absorption and storage.17 Besides, many genera of lactic acid bacteria belong to Firmicutes, they are generally considered beneficial microorganisms associated with a healthy intestinal epithelium.18,19 Proteobacteria is a common dominant bacterial genus in the intestine of aquatic animals,17 like European eel13 and turbot (Scophthalmus maximus L.).20 The increased abundance of Proteobacteria might be associated with the healthy metabolic state, Proteobacteria members have been reported to decompose chemical compounds that can serve as a source of energy and metabolites and improve the health status of fish.21 In the present study, the European eel in the MCE group had lower abundances of Spirochaetae and Fusobacteria, which might be associated with diseases in some aquatic animals.22,23 Thus, MCE supplementation might decrease the relative abundances of those potential pathogens in the intestine of European eels.
In the present study, the relative abundances of Streptococcus and Legionella were significantly higher in the intestine of European eels from the control group. It was reported in aquaculture that some of the well-known fish diseases were caused by Streptococcus species.24 Streptococcus is a well-known pathogen in tilapia culture and cobia fish, which cause mass mortality with significant economic losses.25 Legionella was an opportunistic pathogen.26 Those results indicated that European eels in the control group might be more likely to be infected by some pathogenic species. It was found that there were higher relative abundances of Bacillus, Anaerobacillus, and Sphingomonas in the intestine of European eels from the MCE group. The probiotic effects of some Bacillus were demonstrated to improve growth performance, digestive enzyme activity, and immune response.27,28 In addition, studies have shown that antagonistic effects of Bacillus on the growth of pathogenic Vibrio haemolyticus, Vibrio harveyi, Streptococcus indicus, and Streptococcus agalactiae have been described and initially examined.24 Anaerobacillus is a member of the Bacillus genus and a genetically diverse bacterial species adapted to various environmental circumstances.29 Members of Sphingomonas are of biotechnological interest due to their degradation capabilities for various xenobiotic substances, as well as their potential to produce useful exopolysaccharides and carotenoids such as β-carotene and nostoxanthin.30,31
In the present study, the beneficial change in the intestinal microbiota of European eels was caused by dietary MCE supplementation. Similar results were found in the studies of MCE supplementation in the diets of the American eel,3,10 red tilapia,7 koi carp,1,8 Pacific white shrimp (Litopenaeus vannamei),32 and loach (Misgurnus anguillicaudatus).33 In vitro, it was found that the minimum inhibitory concentration of the main reactive component of MCE (sanguinarine) was in the range of 12.5–50 μg/mL against fish pathogenic bacteria (Aeromonas hydrophila, A. salmonicida, Vibrio anguillarum, and V. harveyi).34 The antimicrobial activities of MCE might be explained by restraining cytokinesis of both gram-positive and gram-negative bacteria by inhibiting Z-ring formation without affecting nucleoid segregation and inhibiting the assembly of purified filamentous temperature-sensitive protein Z, and reducing the binding of filamentous temperature-sensitive protein protofilaments.4,35 It was also reported that alkaloids of MCE had selective effects on harmful microbial growth along the digestive tract.4,10 Those results suggested that MCE might inhibit the action of harmful bacteria in the intestinal wall and reduce the production of toxic compounds and avoid damage to intestinal epithelial cells, thereby protecting the intestinal mucosa and improving the growth performance of some aquatic animal species, including Koi carp,1,8 grass carp,9 Pacific white shrimp,32 and red tilapia.7 The improvement of the growth performance might be achieved under the practical culture condition of European eels, which should be confirmed in the future study.
In conclusion, dietary 100 mg/kg MCE supplementation could beneficially modulate the microbiota in the intestine of European eels cultured in cement tanks.
Acknowledgments
This research was supported by the Regional Development Project for Science and Technology Plan Program of Fujian Province (2022N3002) and the earmarked fund for the China Agriculture Research System (CARS-46). The authors thank Huai-ning Zhu for his assistance with the feeding trial.
Authors’ Contribution per CRediT
Project administration: Gui-hong Chen (Equal), Feng Xi (Equal). Formal Analysis: Gui-hong Chen (Equal), Shao-wei Zhai (Equal). Investigation: Gui-hong Chen (Equal), Shao-wei Zhai (Equal). Writing – original draft: Gui-hong Chen (Lead). Funding acquisition: Feng Xi (Equal), Shao-wei Zhai (Equal). Resources: Feng Xi (Equal), Shao-wei Zhai (Equal). Conceptualization: Shao-wei Zhai (Lead). Methodology: Shao-wei Zhai (Lead). Writing – review & editing: Shao-wei Zhai (Lead). Supervision: Shao-wei Zhai (Lead).