Introduction
Micropterus salmoides, commonly known as Largemouth bass, is native to the freshwater waters of North America. In the 1980s, it was introduced as an alternative to Japanese bass in the country,1 and it began to be successfully artificially reproduced in 1985. With the development of China’s aquaculture industry, largemouth bass has become one of China’s essential freshwater-farmed economic fishes in recent years. In 2016, freshwater cultured largemouth bass production had reached nearly 400,000 tons.
Largemouth bass ranavirus (LMBV),2 Siniperca chuatsi rhabdovirus.3 Infection spleen and kidney necrosis virus4 were the three most common viral pathogens that infect largemouth bass. Among them, the LMBV belongs to the Iridoviridae family Ranavirus, which were nucleocytoplasmic large dsDNA viruses.5 The virus particles were in the shape of a regular hexahedron with a diameter of approximately 140-200 μm. It mainly infects largemouth bass and smallmouth bass, causing large-scale deaths in largemouth bass aquaculture. In China, the LMBV was first reported in Guangdong province in 2008 and has since spread across various provinces, with a rising infection rate and death rate.6,7 In 2021, a new largemouth bass iridescent virus genotype was detected in the southwestern China.8,9 Due to the wide variety of iridoviridae viruses and similar pathogenic mechanisms, people currently do not have a systematic and effective way to control the virus, which is a significant challenge for the breeding industry in China.10
In addition to viral pathogens, bacteria, particularly the genus Aeromonas, pose a significant threat to largemouth bass aquaculture. The genus Aeromonas belongs to the family Aeromonadaceae; members are gram-negative, polar flagellated, non-spore-forming facultative anaerobic rods ranging from 1–3 μm in size. Among the genus Aeromonas, Aeromonas velvetii and Aeromonas hydrophila were common conditional pathogens in aquatic fish, which can cause secondary infections following infection by other pathogens.11 The infection by A. hydrophilic was characterized as chronic hemorrhagic septicemia with signs of ulceration and fin erosion.12 The disease is chronic for several weeks, with increasing mortality and high cumulative mortality13 ADDIN.
Co-infections in fish can result in complex clinical symptoms and require diverse diagnosis methods, which can be challenging for the common fishery to diagnose and treat. In this study, we report a case of co-infection with the LMBV, Aeromonas Vickers, and Aeromonas hydrophila in Jianyang City, Sichuan Province, China. Through a comprehensive pathological examination, we aim to provide references for diagnosing and treating mixed infections in largemouth bass aquaculture, addressing a significant challenge in the industry.
Materials and Methods
Experimental animals and cells
The diseased bass was collected from a perch farm in Jianyang city, Sichuan province, China. Healthy bass individuals, ranging from 6-8cm, were procured from Chengdu, Sichuan Province, China. These healthy perch were subjected to a week-long acclimation period in aerated freshwater tanks maintained at 25 °C. Five randomly selected fishes were subjected to PCR analysis to validate their health status before experimentation and were confirmed to be free from pathogenic microorganisms. The Epithelioma Papulosum Cyprinid (EPC) cells were cultivated in a medium 199 (Gibco) solution containing 10% fetal bovine serum and maintained at 25 °C.
Sample collection and pathological examination
The diseased fish were transported to the laboratory in a specially equipped fish tank, equipped with an oxygenating pump, to ensure the maintenance of their viability before sampling. Clinical signs were recorded, and the liver, spleen, kidney, and gill tissue samples were collected and stored in a refrigerated unit at -80 °C. Part of the tissue samples was collected in formalin solution for making the pathological examination. In a sterile environment, samples were collected using sterile loops from the liver, spleen, and kidney of diseased fish into the BHI plates and were inoculated at 25℃ for 16h in a constant temperature incubator.
PCR detection
Four fish samples (one sample per fish, containing kidney, spleen, and liver) were selected randomly. The total DNA of tissue samples was extracted according to the instructions of the genomic DNA extraction kit (Tiangen, China). The primers were designed according to the sequence of other LMBV MCP strains in GeneBank of NCBI (Forward: 5 ‘-TTTCGGGCAGC AGTTTTCGGT-3’; Reverse: 5 ‘- CCGTAGTTGGTGGAGCC - 3’). The amplification was performed with a total volume of 25μl containing 12.5μL 2×Mix-reaction buffer, 1μL LMBV-F, 1μL LMBV-R, 1μL DNA template, and 9.5μL dd H2O. PCR products were visualized in 1% (W/V) agarose gel electrophoresis containing SYBR-safe DNA gel stain for 15 min at 120 V under UV transillumination. PCR products were purified (Tiangen, China) and sequenced (TsingKe, China), and the blast function of the NCBI (Basic Local Alignment Search Tool) system was used for comparison.
Isolation of virus
For virology examination, the spleen and kidney tissue samples stored at – 80 °C were pooled and homogenized in Medium 199 containing 2% fetal bovine serum at a ratio of 1:100 (weight to volume ratio). The antibiotics-antimycotic was added in a ratio of 100:1. After freezing and thawing, the tissue homogenate in the refrigerator at - 80 °C three times, then centrifuged and filtered through a sterile 0.22μm filter, add it to freshly prepared monolayer epithelioma papulosum cyprinid (EPC) and incubated in culture medium containing 2% fetal bovine serum at 28 °C. Blind passaging once a week and observing daily cytopathic effect (CPE) development.
Infection experiment
Thirty healthy 6-8cm long basses were selected and raised in a laboratory tank. They were randomly divided into two groups. One group was intraperitoneally injected (IP) with 200 μL LMBV-like (JY) culture solution (104.6 TCID50/mL). And the other group was injected with 200ul of sterile PBS. All fish were examined daily for clinical signs and mortality situation for 14 days. During the experiment, the water temperature was adjusted to 25 °C. Diseased fish were dissected, and tissue samples (liver, kidney, spleen) were collected for PCR detection and virus re-isolation of EPC cells. All animal experiments followed the “Guidelines for Experimental Animals” protocols of the Ministry of Science and Technology (Beijing, China).
Molecular analysis of LMBV-MCP gene
Major capsid protein (MCP) is one of the most conserved gene sequences of ranavirus. Many researchers have used it to analyze the evolution of the ranavirus.14 The sequencing results were searched for homology sequences by Blast of NCBI, downloaded, and integrated with FASTA format. The ordered sequences were sorted using the clustalw (codons) function of MEGA 7.0 software with the bootstrap replications set to 1000. Phylogenetic trees were constructed using proximity methods15 ADDIN.
Bacteria identification
The BHI plates of inoculated tissue samples were cultured in an incubator at 28 °C. After the growth of the dominant colonies, the dominant strains named Y3 and Y4 were purified on the fresh BHI medium by streaking and re-streaking till their phenotypes were identified by Gram staining. Genomic DNA was extracted from isolated bacteria Y3 and Y4 using the Dneasy kit (QIAGEN). They were assayed for 16S rRNA—the specific primers of the 16S rRNA-F/R(27F:5’-AGAGTTTGATCCTGGCTCAG-3’; 1492R:5’-TACGGTTACCTTGTTACGACTT-3’). The PCR products were detected by 1%(W/V) agarose gel electrophoresis and photographed under UV transillumination. PCR products were purified (Tiangen, China) and sequenced (TsingKe, China), and the blast function of the NCBI (Basic Local Alignment Search Tool) system was used for comparison.
Antibiotic susceptibility testing
The disk diffusion method was used to test the susceptibility of positive strains isolated by the BHI inoculating.16 Eight drugs were selected on LB AGAR dishes, including cefaclor, gentamicin, neomycin, doxycycline, polymyxin, cotrimoxazole, enrofloxacin, and azithromycin. Drug sensitivity was assessed by measuring the diameter of the inhibition zone using vernier calipers.
Results
Clinical and anatomical symptoms
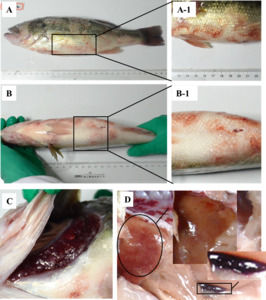
The naturally diseased largemouth bass from the local farm behaved lethargically, lost appetite and poor balance, and swam slowly in the water. The diseased largemouth bass was partly covered with water mold and showed increased mucus secretion on their body surface (Figure 1A). On the body surface, different degrees of extensive ulcerations and necrosis of naked muscle were observed. (Figure 1A-1). Redness was presented in the chest and abdomen surface (Figure 1B) and hemorrhaged at the pectoral and caudal fins (Figure 1B-1). The gill filaments were covered with a small amount of water mold and hyperemic (Figure 1C). The affected fish in this study also showed severe hemorrhages in the liver. The spleen was black, swollen, and had blunt, rounded edges (Figure 1D).
Histopathology
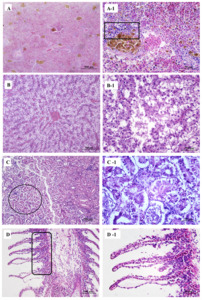
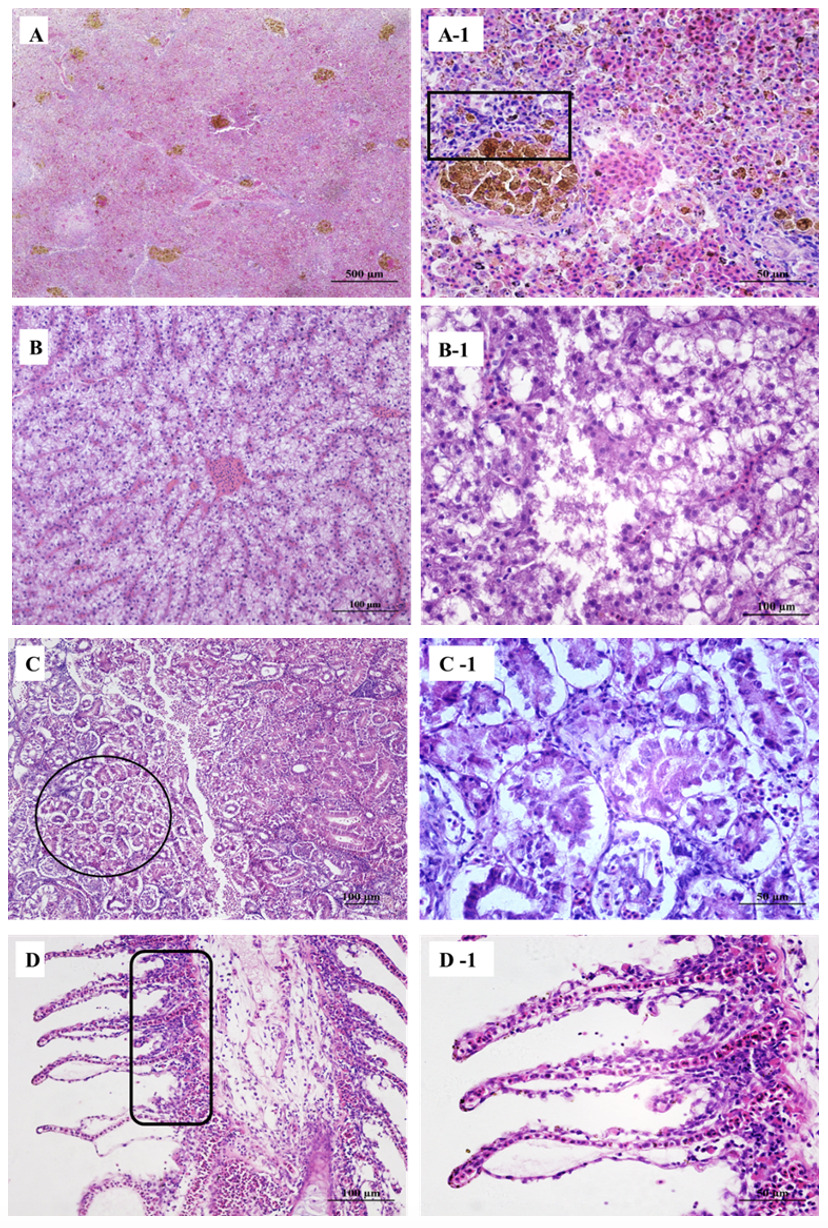
Large hemosiderin deposits were observed in the spleen (Figure 2A) along with vacuolization and dissolution of lymphocytes (Figure 2A-1). The liver showed focal hemorrhages, proliferating lymphocytes (Figure 2B), and multifocal hepatic necrosis spots (Figure 2B-1). The kidney tubules showed vacuolization of cells, narrowing of the lumen, irregular-shaped and abnormally arranged epithelial cells (Figure 2C), surrounded with many infiltrated lymphocytes (Figure 2C-1). Gill artery dilation, congestion (Figure 2D), and gill respiratory epithelium shedding (Figure 2D-1).
Identification of virus
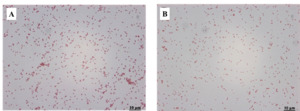
PCR results showed that each of the four randomly selected samples had amplified the characteristic products of 1392bp (Figure 3). After two blind passes of the virus fluid, the typical cytopathological effect (CPE) can be observed, with the cells gradually becoming round, wrinkled, and shedding from the periphery of the monolayer cell, forming numerous empty spots at the bottom of the monolayer cell (monolayer epithelioma papulosum cyprinid Figure 4A), while the control EPC grow densely. No vacuoles appear in the control group (Figure 4B). The infected EPC could also detect positive amplifications using LMBV-MCP primers (Figure 3).
Sequence analysis
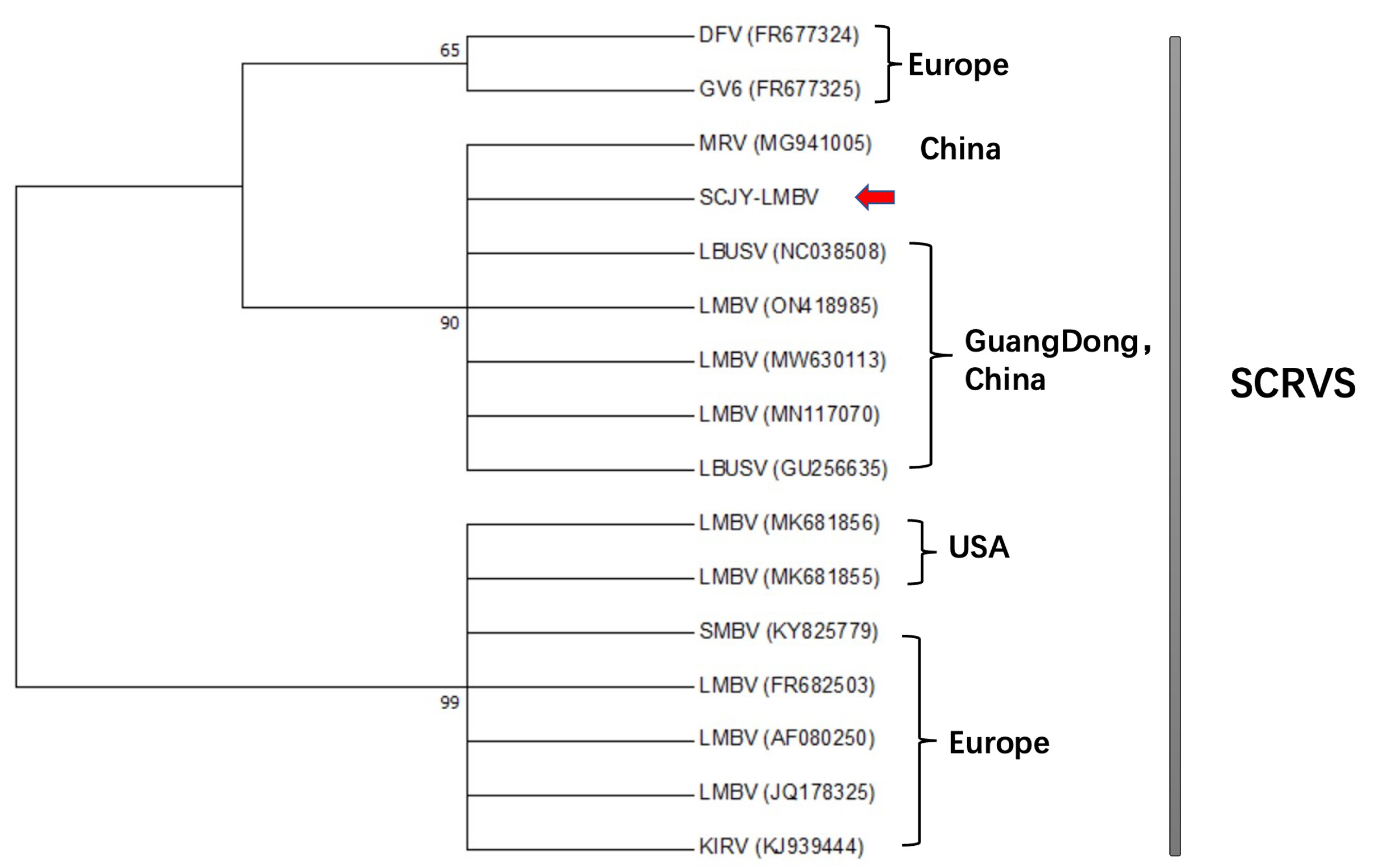
The LMBV-MCP sequence obtained in this study was temporarily named LMBV-SCJY and submitted to Genbank (OP747466). Aligned Sixteen rana viruses to analyze has found that the sequence had the highest similarity with LMBV isolates (ON418985) and (MW630113) from Guangdong Province, China (Figure 5).
Challenge test
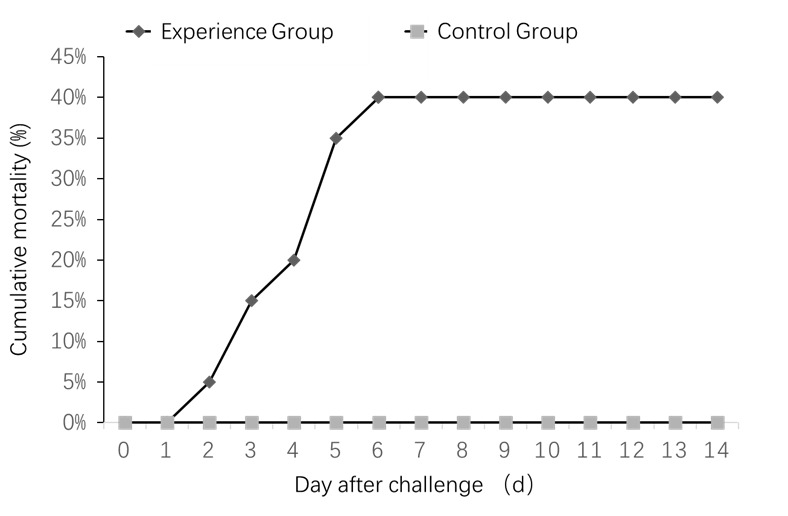
Clinical symptoms began appearing in the experimental group on the second injection day. Death began on day 3, and a cumulative mortality rate of 40% on 6-day-post-infection. Infection in animals was characterized by acute death and has the same clinical symptom as natural infection in fish. The control group survived without abnormalities (Figure 6).
Bacterial isolation and identification
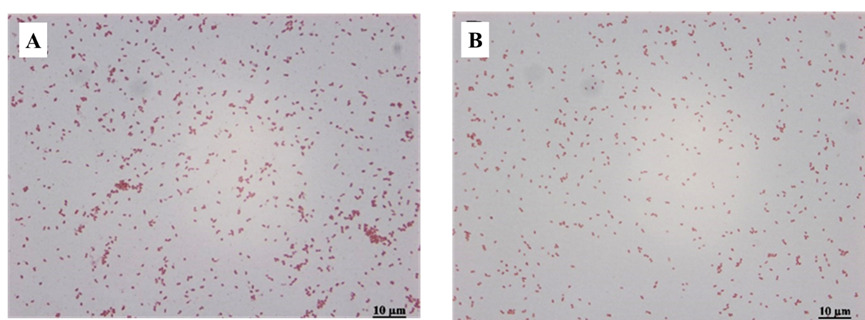
After inoculating BHI plates from diseased fish under sterile conditions and incubating them in an incubator at 28 °C for 24-32h, it was observed that yellowish-white dominant colonies, with a diameter of 2.0 to 3.5 mm, with round, smooth edges and raised centers grew on the inoculated plates. After Gram staining, the dominant colonies showed that the dominant bacteria isolated on the four plates were all adverse reactions with red staining. All of them were scattered with short rod-shaped morphology (Figure 7). The two isolated strains, namely Y3 (Figure 7A) and Y4 (Figure 7B).
After detecting the Y3 and Y4 by 16S rRNA using PCR, they amplified the characteristic products of 1500 bp (Figure 8). The results of Blast alignment on GenBank showed that the sequence similarity between Y3 and Aeromonas hydrophila (MH396747.1) was 99.11%, and the sequence similarity between Y4 and Aeromonas vectori (KU601315. 1) was 99.60%.
Antibiotic susceptibility testing
Kirby Bauer method was used to detect the drug susceptibility of Y3 and Y4 isolates. The results showed that the Y3 strain (Aeromonas hydrophila) was resistant to cefaclor, azithromycin, enrofloxacin, and cotrimoxazole (R), sensitive to gentamicin and doxycycline (S), and moderately sensitive to neomycin and polymyxin B2 (I). The Y4 strain (Aeromonas Vectori) was resistant to cefaclor, gentamicin, neomycin, cotrimoxazole, enrofloxacin, and azithromycin (R), and sensitive to doxycycline and polycolistin B (S)(Table 1).
Discussion
In China, the LMBV is one of the most widespread, prevalent, and highly pathogenic pathogens in largemouth bass farming.6 However, there needed to be an apparent data reference for the genome structure of the LMBV and the epidemic transmission in China. Until now, China has no corresponding national standard to detect the LMBV.
Santi Cooper Raina virus was the primary viral pathogen of seabass infectious canker disease in China, particularly prevalent in high water temperature seasons.9 According to the fish pond owner, water mold fungi began to be seen about three days after the extensive ulcerations and necrosis of naked muscle were observed on the body surface. The largemouth bass died about five days after the onset of typical symptoms. The transmission rate and lethality rate are incredibly high. Ranavirus usually has no distinct characteristic lesions.17 In Western countries such as the United States, the ranavirus typically exhibits a characteristic enlarged and reddened swim bladder.12 The main features of the LMBV found in China include surface ulceration and muscle necrosis (Wu et al., 2020). It was quite different from Western countries. There was no definite conclusion on the reasons for the large gap in the clinical characteristics of LMBV fish in China and Western countries, so it is still necessary to analyze it on a case-by-case basis.
When the host is infected by more than two pathogenic microorganisms simultaneously, it is called co-infection. Many aquatic animals have been reported to be infected by homologous or heterologous pathogens.18 For example, the mixed infection of the leukoplakia syndrome virus and Aeromonas hydrophila in red swamp shrimp in China19 and the mixed infection of tilapia lake virus and Aeromonas viridinosis in Malaysia.20 Co-infection causes worse harm to the host and higher mortality than infection with a single pathogen.18
The largemouth bass in this study has typical muscle ulcer symptoms. The swim bladder had no abnormalities. However, extensive bleeding of organs, enlarged and white liver, and many bleeding spots were seen. The spleen becomes soft, swollen, and black, and a large amount of hemosiderin deposits can be seen under histopathology. Renal function was severely damaged, and tubular epithelial cells were shedding in large quantities. In this study, diseased largemouth bass from a fish pond in Jianyang City, Sichuan Province, was identified as co-infected with LMBV, Aeromonas hydrophila, and Aeromonas vickery. The presence of Aeromonas hydrophila and Aeromonas vicarial was determined by HE staining and PCR. The venom was isolated by cell culture. Through the sequence analysis of the MCP gene of the virus, it was found that the MCP gene sequence of this strain was very close to several LMBV strains in Foshan, Guangdong, China. Still, the gap was evident with several strains in Sichuan, China. This is a question worth exploring for epidemiological investigations.
In the artificial infection experiment, the final fatality rate was 40%. However, the fatality rate of diseased largemouth bass in fish ponds was over 40%. LMBV, Aeromonas hydrophilus, and Aeromonas vickerii can all cause reduced host immunity.18 The LMBV mainly causes a decline in the function of various organs. On the other hand, the common Aeromonas hydrophila and Aeromonas vicarica mainly cause host intestinal infections. Therefore, it can be inferred that the reason for the higher fatality rate in fish ponds is due to the result of co-infection. In conclusion, co-infection with mixed pathogens can cause more severe diseases and higher mortality rates in largemouth bass, resulting in significant economic pressure. Further research is required to explore the pathogenesis of co-infection and develop preventive and treatment measures.
Acknowledgments
This work was supported by Sichuan Science and Technology Innovation Cooperation Foundation (grant numbers 2020YFH0156 and 2022YFN0012) and Sichuan Science and Technology Department (grant number 2021YFN0123).
Author Contribution per CRediT
Conceptualization: Ping Ouyang; Methodology: Jianing Wu; Formal analysis and investigation: Yankai Li, Wenyan Wei; Writing - original draft preparation: Yankai Li, Yongqiang Ren, Shuya Liu; Writing - review and editing: Yankai Li, Ouyang Ping, Xiaoli Huang, Yi Geng, Defang Chen; Funding acquisition: Ouyang Ping, Wenyan Wei; Resources: Xiaoli Huang; Supervision: Yi Geng.

_(lane_m__dna_marker__.png)



.png)
_(lane_m__dna_marker__.png)

.png)