Introduction
Lactococcus garvieae, a Gram-positive bacterium of the genus Lactococcus in the family of Streptococcaceae, is a causative agent of lactococcosis causing systemic hyperacute septicemia in various fish species throughout the world. In addition, as well known as a source of infection for fish, L. garvieae is also considered as a zoonotic pathogen capable of infecting humans.1 Since the first description of L. garvieae involved in septicemia in rainbow trout (Oncorhynchus mykiss) in Japan, the rapidly increasing reports about L. garvieae infecting fish indicates that the fish culture industry has been suffering from significant economic losses due to the outbreaks of L. garvieae disease. In 2018, L. garvieae was first reported to be associated with fatal hemorrhagic septicemia in farmed rainbow trout in India.2 Moreover, tilapia cultivated in Lake Kariba was also strongly affected by the outbreak of lactococcosis.3,4
Golden pompano (Trachinotus ovatus), a kind of economically important marine fish species, is mainly distributed in the subtropical and tropical sea areas of Southeast Asian countries5 and is an economically crucial cultured fish species in China with the production of 239,000 tons in 2021.6 In recent years, the frequent disease occurrence of T. ovatus has caused serious economic losses due to overcrowding, extreme water temperatures, poor water quality, and nutrition in the fast development of the intensive aquaculture.7–9 One common way of controlling lactococcosis is to use antibiotics. Still, the drug residues resulted in the risks for ecological environment pollution, aquatic product safety, and the emergence of drug-resistant bacterial strains. By contrast, a vaccine with higher biological safety is a promising alternative to prevent diseases and reduce reliance on antibiotics.
The most widely used vaccines in aquaculture include subunit vaccines, inactivated vaccines, DNA vaccines, live attenuated vaccines, and genetically engineered vaccines, which can stimulate the fish’s immune system and protect fish against pathogens. Particularly, there is growing interest in developing inactivated vaccines because they are easy to prepare with high efficiency and low cost. Several studies showed that the inoculation with inactivated L. garvieae vaccine offered significant protective efficacy in Nile tilapia (Oreochromis niloticus), rainbow trout (Oncorhynchus mykiss), and Thread-sail filefish (Stephanolepis cirrhifer) against L. garvieae infection.10 These studies indicated that the usage of inactivated vaccine is a potential alternative to address the issue of lactococcosis. However, to the best of our knowledge, there is not yet a commercially available protective vaccine for T. ovatus so far. Therefore, developing innovative inactivated vaccines to protect the T. ovatus against L. garvieae infection is highly demanded in future works.
To enhance the efficiency of inactive vaccines, the adjuvant is often used as a type of helper substance to enhance the immunogenicity of the antigen and prolong the time of antigen release.11 Based on the sources, four types of adjuvants have been used in the fish vaccine, including oils and minerals (mineral oil, Freund’s adjuvant, Montanide, aluminum adjuvant), biochemical agents (interferon, immune RNA, thymus hormone, cytokines), adjuvant from the plant (glycoside, polysaccharide, astragalus polysaccharide), and adjuvant from microorganisms (glucan, flagellin).12 Among these adjuvants, Montanide™ ISA 763A VG (763A) is a type of non-mineral oil emulsion adjuvant with the role of antigen storage and has been used in experimental vaccines and proved to improve the immunoprotection in combination with bacterial antigen.12,13 However, the application of 763 A to the T. ovatus vaccine has not been reported. It remains unclear whether such a chemical adjuvant could improve the efficiency of the L. garvieae vaccine for T. ovatus.
In the present study, we developed and investigated the protective efficacy of the inactivated vaccines (aqueous vaccine and vaccine+763A) against L. garvieae infection in T. ovatus. Meanwhile, we also evaluate the immune response by quantifying the antibody titer, serum enzyme activity, and expression of the immune-related gene in tissues of T. ovatus.
Materials and Methods
Fish and cultural conditions
The healthy T. ovatus weighing 60±15 g was purchased from a commercial fish farm with no history of disease in Zhanjiang City, Guangdong province of China, and maintained at 28.00 ±2.0 °C in floating net cages of the fish farm with the constant and effective water flow. Fish were fed with commercial feed twice daily and acclimatized for two weeks. Before the experiment, to ensure the fish were free of specific pathogens, 10 fish were randomly collected, and parasitological, viral, and bacteriological analysis as described by Wang et al.13
Preparation of inactived L. garvieae vaccine
The L. garvieae strain used in this study was isolated from naturally infected T. ovatus obtained from Zhanjiang City, then identified by biochemical and molecular characterization and stored in the laboratory. L. garvieae was grown in brain-heart infusion agar (BHIA) (Qingdao Hope Bio-Technology Co., Ltd., China) with 5% sheep blood at 30 °C for 48 h. Several colonies were selected from BHIA, inoculated in BHI broth (Qingdao Hope Bio-Technology Co., Ltd., China), and cultured at 30 °C for 24 h. After harvesting, the bacterial cells were killed by adding formalin to a final concentration of 0.3% and incubated at 30 °C for 24 h. Before the formalin addition, the number of bacterial cells was determined by the plate counting method on BHIA. After complete inactivation, the bacterial cells were collected by centrifugation for 10 min at 4 °C and washed three times with sterile phosphate-buffered saline (PBS, 0.01M, pH 7.4) to remove formalin, and then were resuspended with sterile PBS and adjusted to a final concentration of 1.0 × 1010 CFU/mL. No growth was observed by incubating the resuspended bacterin on BHIA with 5% sheep blood at 28 °C for 48 h confirming that the bacterial cells were completely inactivated. The aqueous vaccine was then prepared by diluting bacteria at a final concentration of 1.0 × 109 CFU/mL in PBS. The vaccine combined with adjuvant was prepared by mixing bacterin with adjuvant Montanide™ ISA 763 A VG (Seppic, France) in a ratio of 30:70 using a Test-tube disperse (Tube Drive P control, Germany) to obtain a final concentration of 1.0 × 109 CFU/mL. Similarly, the adjuvant alone for injection was prepared by 70:30 dilution of Montanide™ ISA 763 A VG with PBS as described above. Vaccine safety was evaluated by intraperitoneal (i.p.) injection of T. ovatus with 0.2 mL of aqueous vaccine (1.0 × 109 CFU/mL).
Vaccination and sampling
The healthy fish were randomly selected and divided into 4 groups: group PBS (control), group ISA 763 A, group Lg and group Lisa 763 A. Each group was established with three replicates of 120 fish. The fish in each group received i.p. injections as follows: group PBS: 0.1 mL of PBS, group ISA 763 A: 0.1 mL of adjuvant ISA 763 A only (dilution 70:30 with PBS), group Lg: 0.1 mL of antigens in PBS (aqueous vaccine), Lg+ISA 763 A: antigens mixed with ISA 763A (763A+vaccine).
Fish sampling was conducted weekly from 4 groups for 8 weeks post-vaccination. Following anesthesia with MS-222 (100 mg/mL), blood from 15 fish per group (5 fish/cage) for enzyme activity and antibody detections was drawn from vena caudalis of fish using a sterile syringe at each sampling time points, kept at 4 °C overnight, centrifuged at 3000 g for 10 min (4 °C). The serum was collected and stored at -80 °C until use. Simultaneously, the liver, spleen, head kidney, and intestine tissues were also sampled from fish and preserved in liquid nitrogen for immune-related gene expression analysis.
Challenge test
At 8 weeks post-immunization, challenge experiments were performed using homologous L. garvieae. Briefly, the L. garvieae strain was revived in BHIA with 5% sheep blood at 30 °C for 48 h. The growing single colonies were inoculated into BHI broth and culture at 30 °C for 18 h. The concentration of bacterial cells was adjusted to 1.5 × 108 CFU/mL with PBS. The fish from all groups were injected i.p., with 0.1 mL of live bacterial cells. After injection, all fish were maintained at 30 °C under the abovementioned conditions. The clinical signs, morbidity, and mortality were continuously monitored and recorded for 14 days. To confirm the morbidity caused by L. garvieae infection, the brain, head, kidney, and spleen were removed from the moribund fish under aseptic manipulation conditions for bacteriological examinations. The protective efficacy of the vaccine was evaluated by determining the relative percent of survival (RPS) using the formula: RPS = 1-(% mortality in vaccinated fish/% mortality in control) × 100.14
Agglutination tests
The antibody titers of vaccinated fish were determined by microagglutination test in 96-well hemagglutination plates. Briefly, 50 μl of PBS and 50 μl of serum collected from vaccinated T. ovatus were added in the first well of each row. After mixing thoroughly, 50 μl of diluted serum was two-fold serially diluted into the remaining wells of the same row. Positive and negative serum from T. ovatus were used as controls. Finally, 50 μl of bacterial antigen suspension (at a concentration of 1.0×108 CFU/mL in PBS) was mixed thoroughly to each well. The hemagglutination plates were covered with plastic wrap and incubated in a humidified incubator overnight at 37 °C. Wells were subsequently examined visually and microscopically. The formation of a round diffusion button with fuzzy edges at the bottom of the well in the highest serum dilution was considered a positive reaction. In contrast, the appearance of a round compact precipitate with a clear outline was evaluated as an adverse reaction.
Serum enzyme activity
Enzyme activities of Lysozyme (LZM), superoxide dismutase (SOD), and catalase (CAT) were detected by the detection reagent kits according to the instructions of the products. LZM assay kit (Cat. No. A050-1-1), SOD assay kit (Cat. No. A001-3), and CAT assay kit (Cat. No. A007-1-1) were purchased from Nanjing Jiancheng Bioengineering Institute of China. Each test was replicated in triplicates.
RNA extraction, cDNA synthesis, and quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from the spleen tissue of fish from all groups. According to the manufacturer’s instructions, this process was carried out using RNAprep Pure Tissue Kit (TIANGEN, China). Genomic DNA was removed by incubation at 37 °C with RNase-free DNase I (Takara, China), and purity and concentration of RNA were determined using a NanoDrop2000 spectrophotometer (Thermo, USA). The integrity of RNA was assessed by electrophoresis on 1.2% (w/v) agarose gels.
The purified RNA of each tissue was reversely transcribed into cDNA using EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen Biotech, China). A total reaction volume of 20 µl was performed in a PTC-200 thermocycler (Bio-Rad, USA), which contains 1 µg RNA, 5× reaction buffer 4 µl, 10 mM dNTP Mix 2 µl, random primer 1.0 µl, oligo (dT) primer 1.0 µl, RNase inhibitor 1 µl, M-MuLV Reverse Transcriptase 1 µl, and 8 µl RNase free ddH2O. The reaction conditions were as follows: incubation at 42 °C for 60 minutes and heating at 85 °C for 5 minutes. After the reaction, the cDNA was stored at -80 °C until use.
The synthesized cDNA was diluted 10-fold using the DNase/RNase-free water as a template. The expressions of the immune-related genes IL-1β, TNF-α, IL-8, MHC-Iα, MHC-IIα, CD4, CD8α, and IgM, at different time points were detected by qRT-PCR using PerfectStart® Green qPCR SuperMix kit in LightCycler Real-time PCR detection system (BioRad, USA). All primers used for qRT-PCR were listed in Table 1, and the β-actin gene in each tissue was used as internal control. The reaction volumes of amplification including 5 μL SYBR Green I, 1 μL diluted cDNA, 0.5 μL forward primer and 0.5 μL reverse primers, and 3.0 μL ddH2O. The reaction conditions of qRT-PCR were as follows: 95 °C 5 min; 95 °C 30 s, 60 °C 30 s and 72 °C 30 s for 40 cycles. The relative mRNA transcript levels of the immune-related genes were normalized to β-actin and calculated using the 2-ΔΔCt method. All samples were analyzed in triplicate.
Statistical analysis
The data were presented as means ± standard deviation (SD). Statistical analysis was carried out by one-way ANOVA followed by LSD and Duncan’s test to compare the difference in the relative percent survival, serum-specific antibody titer, serum enzyme activity, and the relative expression of the immune genes among 4 experimental groups in the same sampling time points. The difference was recognized as statistically significant (P < 0.05), and significant differences in inter-group are marked with different lowercase letters (a, b, c, and d). All tests were performed using SPSS 20.0 for Windows (IBM, USA), and the data were plotted using GraphPad Prism 9.0 (GraphPad Software Inc., CA, USA).
Results
Survival the following challenge
During 8 weeks post-vaccination, neither morbidity nor mortality was observed for all fish. In the challenge test, mortalities in the PBS group and adjuvant ISA 763 A group were first observed on 2nd-day post-challenge, increased rapidly on day 3, and continued until day 8 (Figure 1). In contrast, mortalities in Lg and Lg+ ISA 763 A groups were significantly lower than in PBS and ISA 763 A groups. The diseased fish showed clinical signs, including loss of appetite, whirling near the water surface, drifting away from the group, or wandering alone on the water surface. The most apparent gross pathological changes were brain congestion, exophthalmos, periocular congestion, abdomen distension, congestion, and ulceration of the fins. The mortality in the PBS, ISA 763 A, Lg, and Lg+ISA 763 A groups were 96.7%, 88.9%, 17.36%, and 10.0%, respectively. Only two vaccine preparations (aqueous vaccine and ISA 763 A+vaccine) conferred a high level of protection in T. ovatus against L. garvieae, showing RPS values of 90.8% and 80%, respectively. All fish vaccinated with adjuvant+antigen and antigen alone showed significantly increased survival compared to PBS and ISA 763A groups (P < 0.001).
Serum antibody agglutination tier
As shown in Figure 2, the antibody agglutination titers of all immunized groups (Lg and Lg+ISA 763 A groups) were significantly higher than those of PBS and ISA 763 A groups (P < 0.05). The antibody levels increased in all immunized groups at 1-week post-vaccination and peaked at the third-week post-vaccination. Overall, antigens with ISA 763A could effectively increase the serum antibody titers. The antibody titers in the Lg group reached the highest level (1:64) in the 3rd week and turned to decline since the 4th week, while the highest antibody titer (1:128) was observed in the 4th week in the Lg+ISA 763A group. The antibody titers remained above 1:16 in both Lg and Lg+ISA 763A groups at 8 weeks post-vaccination.
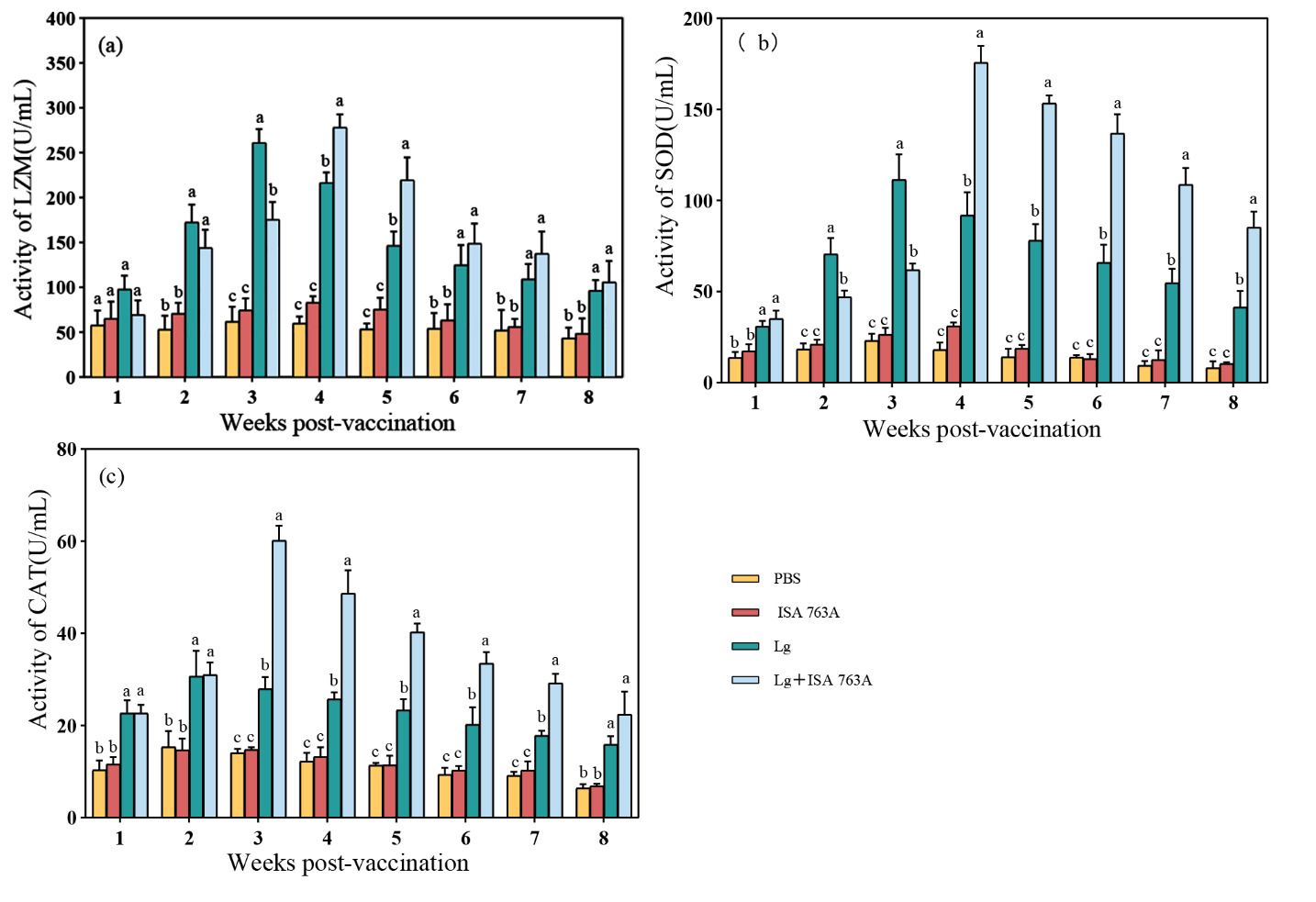
Serum enzyme activity
No significant differences were found in serum LZM activity between ISA 763A and PBS groups (P > 0.05). The LZM activity in Lg and Lg+ ISA 763 A groups was significantly higher than that in ISA 763 A and PBS groups (P < 0.05) from 2 weeks post-immunization and reached the peak value at the 2nd and 3rd week (P < 0.05), respectively (Figure 3). The LZM activity in the Lg+ISA 763 A group was significantly higher than in the Lg group (P < 0.05). SOD activity in Lg and Lg+ISA 763A groups increased from 1-week post-immunization, reached the highest level at the 3rd and 4th week, respectively, and were significantly higher than that in ISA 763 A and PBS groups (P < 0.05) at all sampling time-points (Figure 3). The SOD activity in the Lg+ISA 763 A group was also significantly higher than in the Lg group (P < 0.05). Fish in Lg and Lg+ ISA 763A groups showed significantly increased serum CAT activity compared to that in PBS and ISA 763A groups from 1-week post-vaccination (Figure 3). The CAT activity peaked at the 2nd week in the Lg group and the 3rd week in the Lg+ISA 763A group, respectively.
Expression of the immune gene in spleen tissue
The mRNA transcript levels of IgM, MHC-Iα, MHC-IIα, CD4, CD8, IL-1β, TNF-α, and IL-8 genes in the spleen during 8 weeks post-immunization were analyzed by qRT-PCR. Low levels of expression of all examined genes in the PBS and ISA 763 A groups were detected. Significant expression levels were observed in Lg and Lg+ISA 763A groups throughout 8 weeks post-immunization (Figure 4). The general trend of gene expression of all examined genes in the spleen was very similar first increasing and then declining. In comparison with the expression in PBS and ISA 763 A groups, IgM, MHC-Iα, MHC-IIα, CD4, CD8, IL-1β, TNF-α, and IL-8 were significantly up-regulated in the vaccinated T. ovatus throughout the experiment (Figure 4).
Discussion
Although some chemotherapies have shown high antimicrobial activity against L. garvieae in vitro, developing vaccines and vaccination is essential to control lactococcosis.15 To date, several lactococcal vaccines with/without different adjuvants have been reported to show strong immunoprotective effects on fish through injection and oral methods, and there is a compelling commercial fish vaccine against L. garvieae infection in Europe.16–18 Reasonable protections (RPS > 80%) were usually obtained at 3 weeks post-vaccination with bacterins alone and 4–5 weeks post-vaccination with vaccines combined with oil-adjuvant.19,20 However, the duration of protection is often shorter than 3 months with water-based vaccines and last for 5-8 months with adjuvant vaccines in rainbow trout.20 Similarly, the aqueous vaccine of L. garvieae yielded an RPS of 90.8% at 8 weeks post-vaccination in the study, indicating that the inactivated bacterin has good immunogenicity. Moreover, the application of the vaccine combined with ISA 763A increased the RPS to 90.8%, suggesting the oil adjuvant can effectively improve the effect of inactivated bacterin against L. garvieae on T. ovatus. The results are consistent with the previous study on rainbow trout.19,20 The possible mechanism is that oil-based adjuvants have a depot effect, which releases antigens slowly into tissues or blood, enhancing and prolonging the immune response.21
In fish, innate immunity is the first line of defense against pathogen invasion before adaptive immunity production. Lysozyme is a bactericidal enzyme by hydrolyzing the β-1, the 4-glycosidic bond between N-acetylglucosamine and N-acetylamino in the peptidoglycan of bacterial cell walls, which enables the enzyme can lyse some G+ bacteria and even G- bacteria together with the complement.22 Antioxidant enzymes, including SOD and catalase constitute the first line of the enzymatic defense mechanism against ROS and maintain cyto-homeostasis and the balance of the immune system.23 L. garvieae vaccine in this study could effectively improve the non-specific immunity of T. ovatus, which was beneficial for T. ovatus to maintain the normal physiological state and remove the invading pathogen. This was supported by inactivated bacterin alone and with ISA 763A significantly increasing levels of CAT, SOD, and LZM in T. ovatus post-vaccination. Humoral immunity plays a significant role in protecting fish against L. garvieae infection. Vaccination using L. garvieae bacterin could effectively promote specific antibody levels in fish.19,20 Likewise, the present study showed that the antibody titers could only be increased by the stimulation of the L. garvieae vaccine and were further increased by the supplement with ISA 763A in T. ovatus. Therefore, these results suggested that the ISA 763A could assist the formalin-inactivated L. garvieae vaccine in eliciting the non-specific and specific immune response to defend against the infection of L. garvieae in T. ovatus. The role of ISA 763A as vaccine additives observed in this study was consistent with previous studies using other fish vaccines.20
The mRNA expression levels of the immune-related genes can evaluate fish-specific immunity and non-specific immune response. IL-1β and TNF-α are two pivotal pro-inflammatory cytokines that regulate the production of other cytokines and potentiate the proliferation, differentiation, and function of diverse immunocompetent cells.24,25 IL-8 was the first discovered chemokine that attracts neutrophils, T lymphocytes, and basophils in vitro, with the biological effects to increase cytosolic calcium levels, respiratory burst, and chemotaxis in neutrophils.26 IL-1β, TNF-α, and IL-8 expression levels significantly increased in Lg and Lg+ISA 763A groups compared to in the PBS and ISA 763A groups. Similar results were also observed in rainbow trout following bath vaccination with a bacterin of Yersinia ruckeri.27 These results suggested that the inactivated L. garvieae vaccine could enhance the innate immune response of T. ovatus and further regulate the expression of other immune-relevant genes.
T cells in fish are classically divided into two general subpopulations based on their function, cytotoxic T cells (CTLs) and helper T (Th) cells. The CTLs expressing CD8 molecules clear cells infected with intracellular pathogens and present peptides on the membrane-bound MHC I molecule. Meanwhile, helper T cells expressing CD4 interact with MHC class II, triggering a Th2- type immune response. This study found significant up-regulation of MHC-Iα, MHC-IIα, CD4, and CD8α genes following vaccination, similar to previous studies using other vaccines.25,28 These indicated that the inactivated L. garvieae vaccines could simultaneously improve the host humoral and cellular immunity. IgM is a specific pivotal component of humoral response in teleost fish and mainly plays a role in engulfing the pathogenic bacteria invading the body and participating in the immune response. Previous studies showed that high expression levels of the IgM gene were achieved in spleen tissue when fish was vaccinated by injection and oral route.29 In this study, IgM gene expression levels were up-regulated in 1st week and reached the highest in 3rd and 4th weeks post-vaccination, respectively. These indicate that activating the adaptive immune system and producing specific antibodies in fish are complex and time-consuming. Expressing immune-related genes after inoculation with L. garvieae vaccines provides a better understanding of the immune mechanism against L. garvieae in T. ovatus. Further studies are needed to elucidate the function of the immune–relevant genes against L. garvieae infection.
In conclusion, the present study indicates that the formalin-inactivated vaccine formulated from antigens alone and combined with oil adjuvant ISA 763A could provide high immune protection of T. ovatus against L. garvieae infection. The vaccine enhances antibody production, serum immune enzyme activity, and immune-related gene expression levels. The ISA 763A adjuvant showed good compatibility with the proposed vaccine. The vaccine could effectively immunize the farmed golden pompano to enhance disease resistance.
Acknowledgments
This study was financially supported by the Zhanjiang Science and Technology Plan Project (2021A05196), the National Key R & D Program of China (2022YFD2401200), Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (ZJW-2019-06), and the Key-Area Research and Development Program of Guangdong Province (2021B0202040002).

_of_*t._ovatus*_in_different_groups_challenged_with_*l._garvieae*_for_14_days.png)



_of_*t._ovatus*_in_different_groups_challenged_with_*l._garvieae*_for_14_days.png)