Introduction
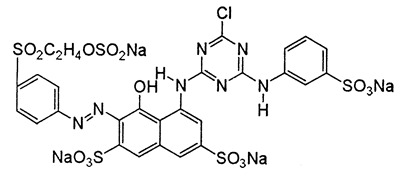
Reactive Red 198 (RR 198; CAS Number 145017-98-7, Molecular Weight: 984.2) (C27H18ClN7Na4O16S5) is among the most commonly used dyes. Reactive dyes are mainly used to dye cotton-based products in the textile industry. Reactive dyes contain dichlorotriazine or monochlorotriazine rings. It is the only dye group that can form a true, strong covalent bond with the functional groups in the fiber structure. The strong binding of reactive dyes with fiber is due to chlorotriazines.1 Its small particle feature penetrates the fiber quickly and provides a vivid, bright coloring.2 The chemical structure of RR 198 is given in Figure 1.
Since most of the reactive dyes that remain in the wastewater after the dyeing process have a toxic effect, removing these colorants from wastewater has become mandatory. Considering the scarcity of water resources, environmental pollution, and the continuation of aquatic life, removing these products is quite important.
There are various physicochemical methods employed to remove dyes from wastewater such as ozonation, biosorption, electrochemical degradation, chemical coagulation, reverse osmosis, cation exchange membranes, ozone treatment, nanofiltration, ultrafiltration, and photochemical oxidation.3 Most dyes are extremely resistant to chemical and biological methods due to their high solubility in water. Adsorption is the most preferred method because it is easy, cheap, clean, and produces less waste afterward.
Adsorption is the transfer, accumulation, and condensation of atoms, ions, and molecules, which are in the fluid phase, to the surface of another phase. The separation process usually occurs on the phase surface and takes place as adhesion. Adsorption occurs at the interfaces in two phases (i.e., liquid-liquid, liquid-solid, liquid-gas, solid-gas) based on the adhesion principle.4 It results from unbalanced forces between the surface molecules of the adsorbent. The forces holding the molecule together are greater than the force pulling the molecule outwards. The imbalance in the forces, which pull the molecule outwards, causes it to attract other substances dissolved in the solvent towards itself. Unbalanced surface forces of the molecule are therefore balanced by this attraction and adhesion (i.e., adsorption) occurs with this stabilization. This phenomenon reduces the surface energy of the system.5–7 The heat emerging during adsorption is the result of the interaction between the adsorbed particles and unbalanced forces on the surface.8
The most commonly used adsorption type in wastewater treatment processes is liquid-solid adsorption. The accumulation of dissolved substances on the water surface depends on the attractive forces between the solvent and the adsorbent.9,10 The nonpolar molecules in the water move toward the interfaces between the adsorbent and the liquid.11 Consequently, adhesion to the adsorbent surface begins and the surface tension of the solvent decreases. The rate of adhesion to adsorbents in refinement processes is very important for the efficiency of the process.12
Recently, studies on chitosan have been carried out as an adsorbent material in adsorption processes, wastewater treatment, and other industrial applications. Chitosan is found in the shells of arthropods such as crab, shrimp, and lobster, as well as in the cell walls of some bacteria and fungi. It is a polymer obtained by deacetylation of chitin (β-(1-4)-poly-N acetyl-D-glucosamine), the second most abundant polymer in nature after cellulose. Chitosan is an important and interesting biomaterial in the pharmaceutical and medical fields, because it has no toxicity, does not cause allergies and irritations, and is biodegradable and biocompatible. Chitosan has found use in many industries, especially in food, cosmetics, agriculture, medicine, paper, and textile.13 In the present study, chitosan was used as the dye adsorbent.
Chitosan is used in the removal of dyes due to its competence to adsorb the reactive dye in wastewater in a wide pH range, and in water conditioning as a coagulant and flocculant due to its cationic polymer property.14,15
Although there are different studies on the removal of different dyes from wastewater with chitosan,16–19 the number of studies on RR198 removal is limited.20 Therefore, the present study aimed to investigate the usability of chitosan obtained from shrimp shells, which are not utilized and possess the potential of serious environmental pollution problems, as an effective adsorbent material in the removal of RR 198 under different experimental conditions. The effects of different pH, temperature, initial dye concentration, and particle size conditions on the adsorption are discussed. The Langmuir adsorption isotherm was used for kinetic modeling.
Materials and Methods
Adsorbent and Adsorbate
In the present study, the chitosan extracted by Küçükgülmez et al.21 was used as the adsorbent. The deacetylation degree of the chitosan was 92.19%.
Reactive Red 198, purchased from Berdan Textiles Company (Türkiye), was used as the adsorbate. Stock solutions were prepared by dissolving the RR198 in distilled water. The desired conditions were obtained by diluting, so aqueous solutions were prepared.
Characterization of the Chitosan
FTIR analysis of the chitosan
Chitosan samples were dried in an oven at 90℃ both before and after the dye adsorption. The infrared spectrum (IR) characterization was conducted in a Bruker Spectrometer (Alpha Platinum, ATR) using 3-4000 cm-1 spectral range.
SEM images of the chitosan
The dried chitosan samples were initially coated with Gold-Palladium (Au/Pd) using an automatic coating machine (Cressington Sputter Quater 108 Auto). SEM characterization was carried out using an FEI (Quanta FEG 250 model) type instrument in a vacuum environment. Images of the sample surfaces were recorded from different areas with different magnifications.
Adsorption tests
Effect of the pH
To determine the effect of the pH on dye adsorption, 8 different solutions (0.3 mM each) at pH values of 2, 3, 4, 5, 6, 7, 8, and 9 were prepared by diluting the stock solution. One g/L chitosan was added to the prepared solutions. The solution was then agitated in an orbital shaker for 24 hours at 120 rpm. Afterward, samples were centrifuged and the absorbance values were read with a UV-VIS spectrophotometer to determine the remaining dye amount in the solutions.
Effect of the temperature
To determine the effect of the temperature on dye adsorption, 100 mg/L dye and 1 g/L chitosan were agitated at 120 rpm and 20, 30, and 60℃. The samples were then read using a UV spectrophotometer.
Effect of the dye concentration
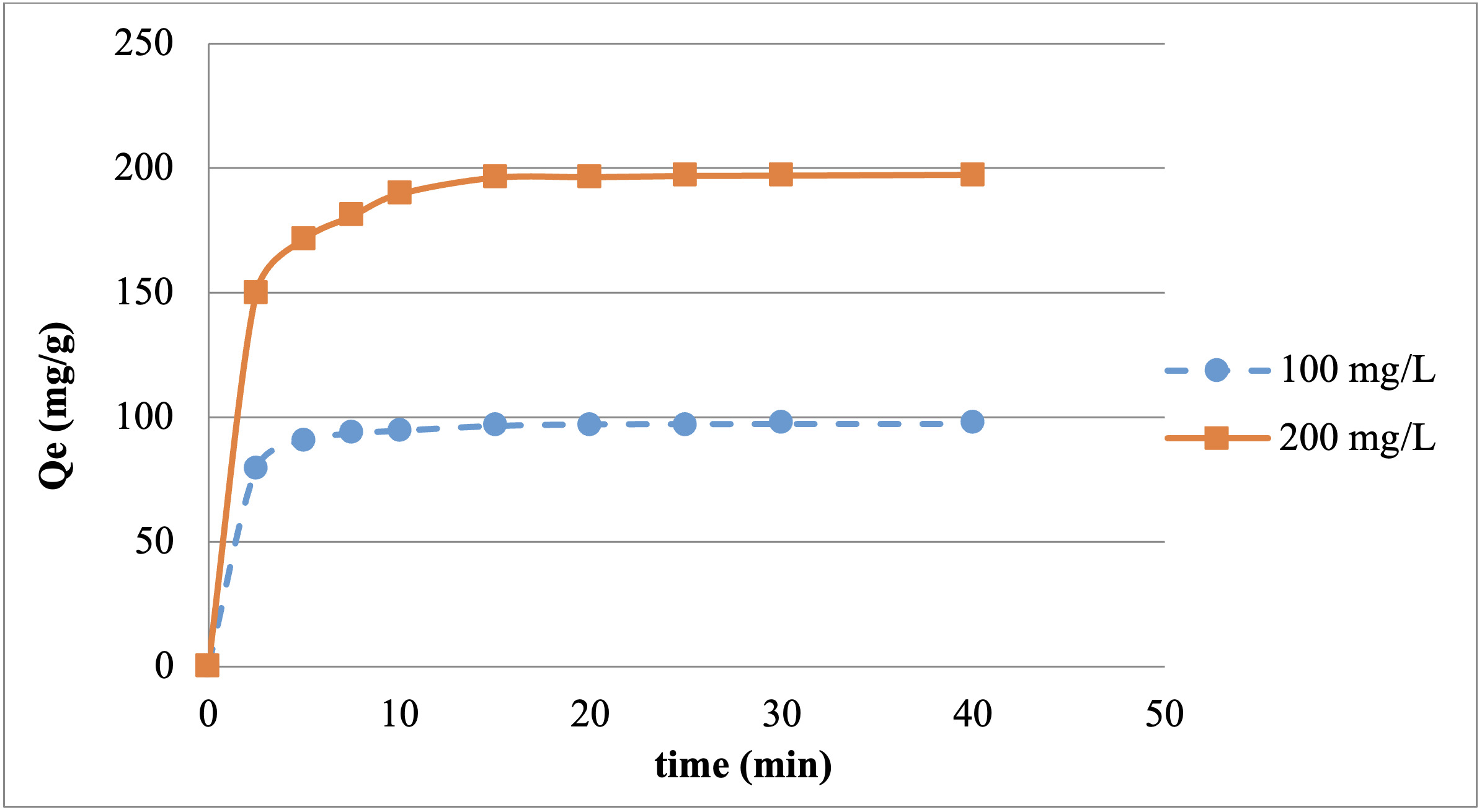
The effect of the dye concentration on adsorption over time was investigated. Two separate solutions of Reactive Red 198 were prepared in the concentrations of 100 and 200 mg/L. The solution’s pH was adjusted to 5, and solid chitosan particles were added to a final concentration of 1 g/L. The mixture was agitated at 120 rpm and 20℃ for 40 minutes. During the contact time, samples were taken at certain intervals (Figure 2), and spectrophotometric measurements were conducted after centrifugation.
Effect of the particle size
Chitosan samples were passed through a 150-mesh sieve, and the samples remaining on each side of the sieve were used in the adsorption trials. Samples retained were named over-size, whereas samples passed named under-size particles. Adsorption was carried out at 120 rpm and 20℃ over time. Spectrophotometric measurements were carried out at certain intervals.
Isotherm Study
The adsorption process was carried out by agitating the dye solutions prepared at different concentrations (including 1 g/L chitosan) at 120 rpm for 3 hours to determine the adsorption isotherm.
Results
Characterization of the chitosan
The FTIR spectra of the chitosan samples before and after dye adsorption are given in Figure 2.
Looking at the FTIR spectra before the adsorption, the peaks between 400 and 650 cm-1 are the peaks that are expressed as fingerprint peaks. Peaks at 1145 cm-1 refer to ring-shaped C-O-C bonds, peaks at 1310-1372 cm-1 to C-CH3 and CH2 peaks, peaks at 1419 cm-1 to stretching vibration peak of CH molecule, peaks at 1572 cm-1 to NH and C-N bond peaks in amide II group, peaks at 1646 cm-1 to C=O ponds in the amide I group, peaks at 2868 cm-1 to stretching vibration peak of -CH, and peaks at 3352 cm-1 to O-H bond peaks.
Electron microscopy (SEM) images of chitosan samples obtained before and after dye adsorption are presented in Figure 3. In the study, the images were taken with different magnifications. SEM is a system for taking high-resolution images from the sample surface using electrons and is used to obtain three-dimensional images of the sample surface.
Effect of the pH
To determine the effect of the pH on dye adsorption, 100 mg/L dye and 1 g/L chitosan concentrations were tested at the pH range of 2-9. The graph regarding the effect of the pH on adsorption is given in Figure 4.
One of the most important parameters affecting the functioning of the adsorption mechanism is pH. The pH value of the solution has a great effect on the adsorbing capacity of the adsorbent. Changes in the pH affect dissolution, ionization, and surface charge of the adsorbent.
As a result of the experiments carried out to investigate the effect of the pH in the adsorption of Reactive Red 198 with chitosan, the adsorption was recorded as 186.7, 193, 196.5, 197.4, 195.7, 194.3, 193.8, and 192.4 mg/g at the pH levels of 2, 3, 4, 5, 6, 7, 8, and 9, respectively.
It was observed that the adsorption begins at pH 2. Still, it is inefficient due to the repulsion between the chitosan molecules due to excessive protonation at this point. The highest adsorption value was obtained at pH 5, which was determined to decrease after this point (the pH range of 6-9).
Effect of the temperature
Temperature is another parameter that affects the adsorption mechanism. The relationship between temperature and adsorption efficiency depends on whether the adsorption is endothermic or exothermic. To determine the effect of the temperature on adsorption, studies were carried out at 20, 30, and 60℃ with 100 mg/L Reactive Red 198 and 1 g/L chitosan. As a result of the experiments, the Reactive Red 198 adsorption was found to be 196.9 mg/g at 20℃, 195.3 mg/g at 30℃, and 191.7 mg/g at 60℃. The temperature-dependent adsorption graphic is given in Figure 5.
Effect of the initial dye concentration and contact time
The time-dependent variation in removing Reactive Red 198 from an aqueous solution with chitosan was examined by sampling at certain intervals. After passing through a 150-mesh sieve, 1 g/L retained (over-size) and passed (under-size) chitosan were separately used with 100 and 200 mg/L dye. Figures 6 and 7 show the Reactive Red 198 adsorption by under-size and over-size chitosan, respectively.
Effect of the particle size
The particle diameter is a very important parameter in adsorption studies as it is directly related to the surface area of the adsorbent. In the study, over-size and under-size chitosan particles were used with the dye concentrations of 100 and 200 mg/L at room temperature.
Rapid adsorption occurred in the first few minutes in both particle sizes. While the under-size particles adsorb better, the adsorption ability of the over-size particles is relatively weak.
Adsorption Isotherm
Isotherms are theoretical calculations made for kinetic modeling of adsorption to be informed about the adsorption process and to be used in engineering studies. The Langmuir isotherm model was employed in this study. Isotherm studies were carried out with different initial dye concentrations (50, 75, 100, 150, and 200 mg/L) and 1 g/L chitosan at room temperature and pH 5.
The Langmuir isotherm parameters for chitosan were found to be aL=0.125, kL=62.5, and Qmax value was computed to be 500 mg/g.
Adsorption Kinetics
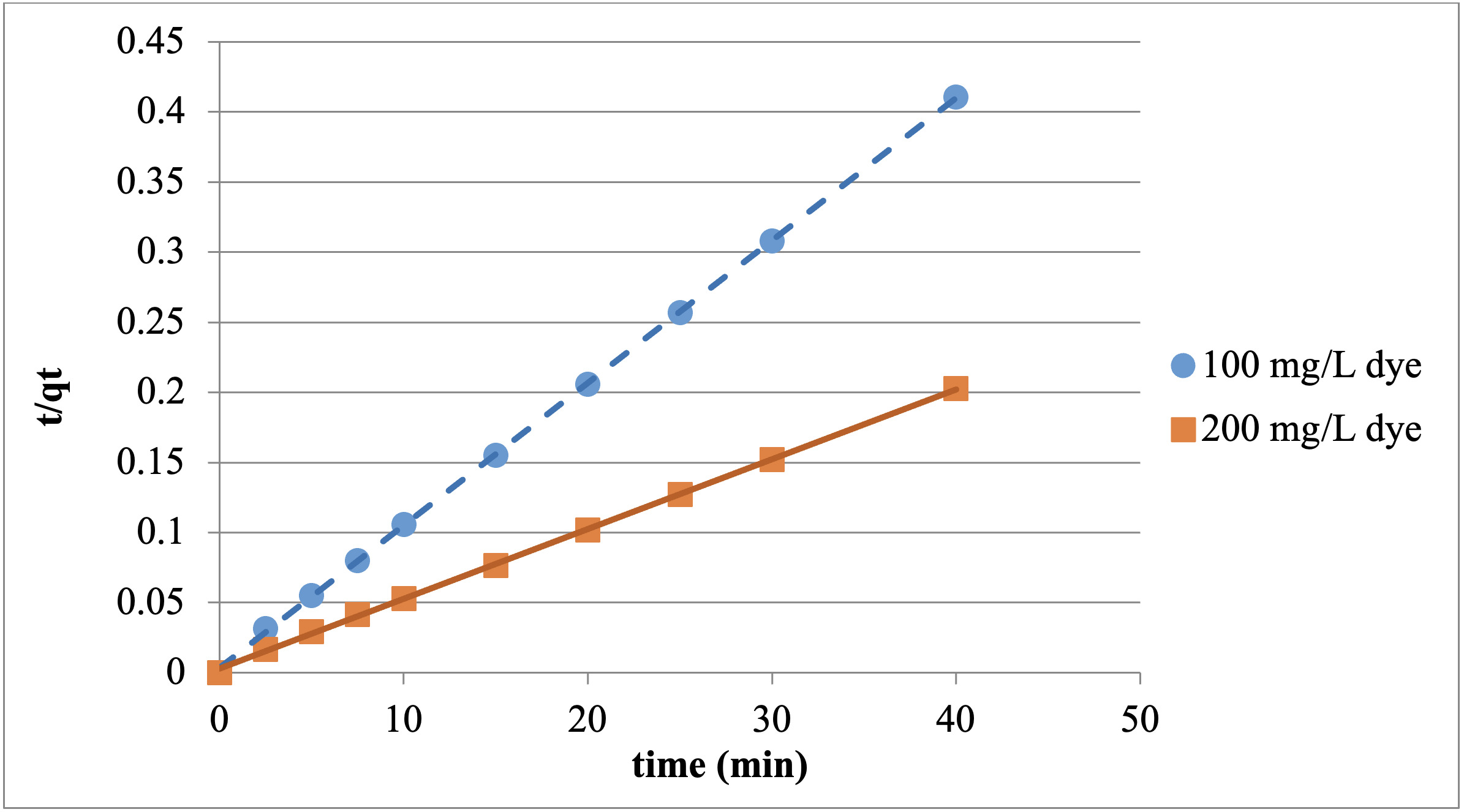
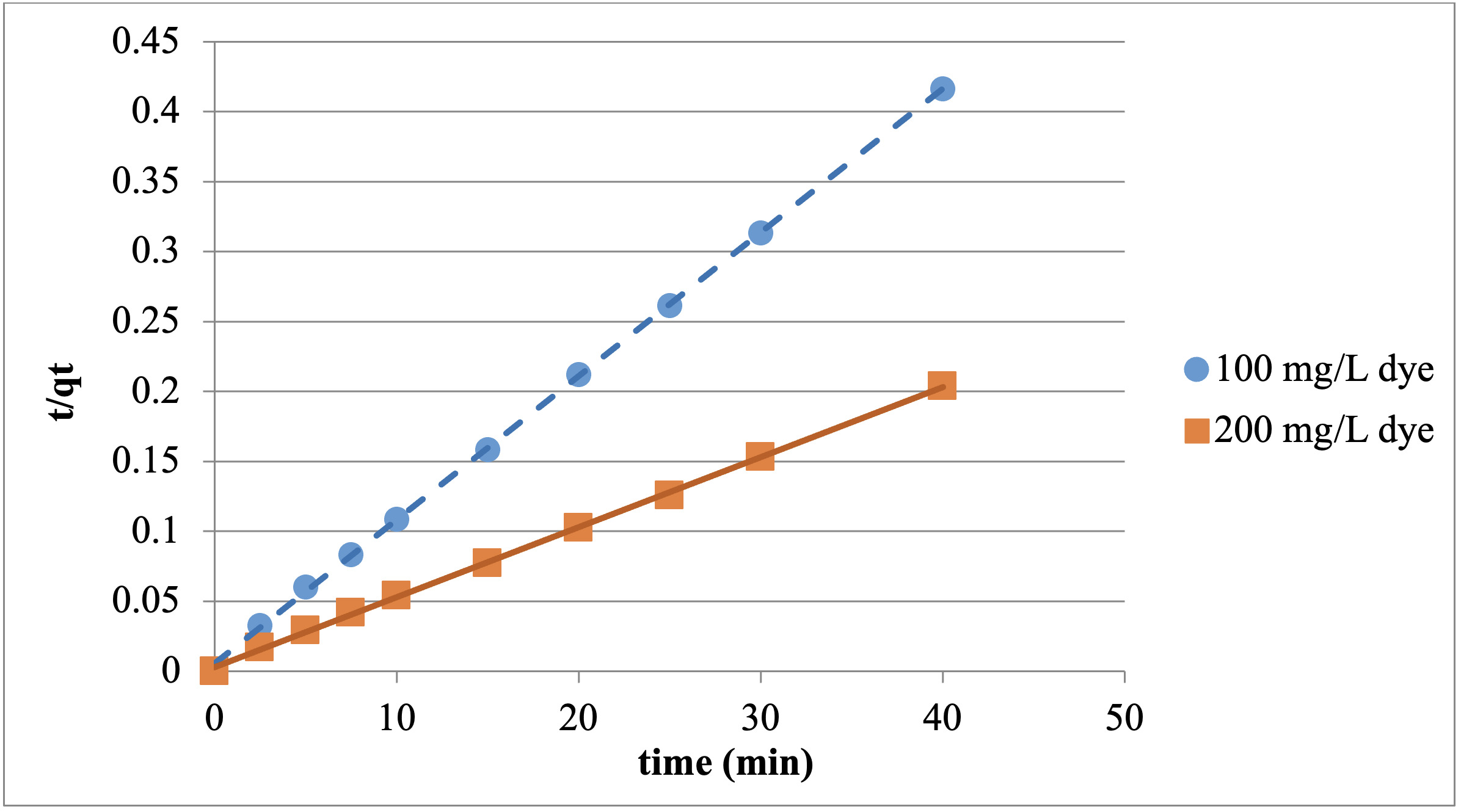
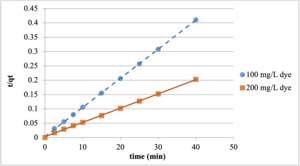
Kinetic modeling is needed to use the adsorption study in engineering calculations, and to understand the feasibility of the system as well as the suitability of the adsorbent. In this study, as a result of the experiments conducted with 100 and 200 mg/L dye concentrations at 20℃ and the minutes 2.5, 5, 7.5, 10, 15, 20, 25, 30, and 40, it was observed that the kinetic modeling for the adsorption of the Reactive Red 198 with chitosan fits the pseudo-second-order kinetic model.
The effect of the Reactive Red 198 concentration on adsorption kinetics was determined using pseudo-second-order kinetic modeling. Temporal adsorption kinetics were investigated with 100 and 200 mg/L dye, at 20℃ and at the contact time of 40 mins for both particle sizes. The graphics for the adsorption studies, which were conducted with chitosan and fit the pseudo-second-order kinetic model, are presented in Figures 8 and 9.
Pseudo-second-order parameters obtained by plotting t/qt against t are given in Tables 1 and 2.
Discussion
In the last decades, water pollution has spread worldwide, especially in underdeveloped and developing countries. Removal from the ground and surface water is a significant global challenge.22 The adsorption method has attracted much attention and is considered the common separation technique due to its ease of design and operation, high removal efficiency even from dilute solutions, and low initial cost.23,24 Among low-cost adsorbents, chitosan and its derivatives have attracted great attention in removing organic and inorganic pollutants from water.25,26
FT-IR spectroscopy is used for the identification of organic compounds. As shown in Figure 2, after the adsorption, the FT-IR peak at 1646 cm-1 changed to 1629 cm-1, the peak at 1572 cm-1 changed to 1533 cm-1, and the percent transmittance values of these peaks increased. On the other hand, the peak at 1419cm-1 changed to 1421cm-1, the peak at 1372cm-1 to 1376 cm-1, the peak at 1310 cm-1 to 1313 cm-1, and the percent transmittance values of these peaks decreased after adsorption. This shows that the Reactive Red 198 forms a chemical bond with chitosan, indicating that the adsorption is chemical. The pseudo-second-order kinetic model is the kinetic model indicating the chemical adsorption, and the FTIR results also support this.
Looking at the SEM image (Figure 3) of chitosan without dye, multi-layered fibrous structures on the surface of the chitosan particles and indented structures on the particles were observed. The forms of the indented and fibrous structure create porous structures on the chitosan particle. This enables the molecule to expand its surface area and open areas that will form the inner surface of the molecule while being used in adsorption studies. When the images of the chitosan that adsorbed the RR 198 were examined, it was seen that dye molecules penetrate from the surface of the layered fibrous structure of the chitosan and adhere by covering the surface. Furthermore, it was observed that the inner surfaces of the pores on the chitosan particle surface and the entrance doors were coated with the dye molecule. As a result, according to the SEM images before and after the adsorption, it was observed that the adsorption took place. Moreover, after the contact of the chitosan molecules with the dye, the adsorption occurred on the outer and inner surfaces. The present SEM results indicate that chitosan particles can be used as a suitable adsorbent in the adsorption of the Reactive Red 198.
One of the most important parameters affecting the functioning of the adsorption mechanism is pH. Since chitosan dissolves in acid, free amine (-NH2) groups in its constitution are protonated after dissolution, and the chitosan gains cationic properties. As the surface of the adsorbent will be positively charged at the pH values below the ZPC (isoelectric point) of the adsorbent, the adsorption of the Reactive Red 198, an anionic dye, will be higher at low pH values. A decrease in the adsorption capacity was observed at the high pH levels. This can be explained by the competition between the hydroxyl (-OH) ions in the alkaline solution and the sulfonate (SO3-) ions in the dye molecule to interact with the cationic chitosan surface. Likewise, a similar study reported that the decrease in the positiveness on the adsorbent surface in an alkaline environment causes a decrease in the adsorption.27 Similarly, Shankar et al.28 stated that the active adsorption sites of the chitosan are secondary hydroxyl (-OH) and primary amine (-NH2) groups, and these amine groups are functional groups that can be easily formed to give chitosan the desired adsorption property. The same study concluded that the feature that distinguishes chitosan from many other adsorbents is the role of its surface during the adsorption process (i.e., acting as a complex environment for ionic types of adsorbates).
Temperature is another parameter that affects the adsorption mechanism. It was observed that the adsorption decreases as the temperature increases. This shows that the adsorption is exothermic. Moreover, this correlation can be explained by the fact that, at high temperatures, the relationship between chitosan and solvent becomes more effective than the relationship between chitosan and dye molecules. As a consequence, chitosan dissolves and the Reactive Red 198 dye adsorption reduces.
Rapid adsorption occurred in the first few minutes in both particle sizes. While the under-size particles adsorb better, the adsorption ability of the over-size particles is relatively weak. This can be attributed to the relationship between the effective specific surface area and the particle size of the adsorbent since adsorption is a surface-related phenomenon. Similar to this study, Guibal et al.29 reported that the smaller the particle size, the more dye was absorbed from the system.
The time-dependent variation in the removal of Reactive Red 198 from aqueous solution with chitosan was examined by sampling at certain intervals. It was observed that the adsorption increases with the increase of the initial dye concentration. There was a steady increase in the adsorption capacity from the beginning. Adsorption occurred rapidly in the first 2.5 minutes. A decreasing increase was observed over time and it took 25 minutes for the system to reach equilibrium for both dye concentrations when under-size chitosan was used. For over-size chitosan, this duration was recorded to be 30 minutes. The reason for the slight time difference is thought to be because the difference between the particle sizes of the studied chitosan is small and the particles are inhomogeneous distribution. After the system reached equilibrium, no significant increase in the adsorption amount was observed. These results show that the initial dye concentration and the particle diameter do not change the reaction’s time to reach equilibrium much.
As can be seen in the table 1 and 2, very high correlation values were obtained for the results of the adsorption kinetics. It was also found that the theoretical and experimental qe values were approximate. This shows that the adsorption fits the pseudo-second-order kinetic model.
Chitosan is a natural polymer, and its high capacity in the adsorption process has shown that it is an applicable material in the industry instead of the synthetic adsorbents used in wastewater treatment. It is the only adsorbent to be used in treatment processes for reducing carbon footprint, which is the main struggle against global warming. In this study, it was inferred that it has a higher adsorption capacity compared to other biological adsorbents.
Since chitosan can respond to adsorption in a wide pH range, its usability in removing heavy metals, pesticides, pharmaceuticals, etc., provides an advantage in industrial applications.
According to the isotherm and kinetic model results, it was concluded that chitosan treatment could be a viable process in dye removal from wastewater. As a result, it was seen that chitosan biopolymer obtained from the shellfish wastes, which are disposed of without utilization, can be successfully used to remove Reactive Red 198, a dye causing pollution in wastewater. The results of the present study will encourage further studies on the removal of other dyes in wastewater
Acknowledgments
This article is part of MSc Thesis of Hamiyet Özge Carbaş.
Author contributions
Investigation: Hamiyet Ö. Carbaş (Equal), Ali E. Kadak (Equal), Aygül Küçükgülmez (Equal). Resources: Hamiyet Ö. Carbaş (Equal), Aygül Küçükgülmez (Equal). Methodology: Hamiyet Ö. Carbaş (Equal), Ali E. Kadak (Equal), Aygül Küçükgülmez (Equal). Formal Analysis: Hamiyet Ö. Carbaş (Equal), Ali E. Kadak (Equal), Aygül Küçükgülmez (Equal). Writing – original draft: Hamiyet Ö. Carbaş, Ali E. Kadak (Equal), Aygül Küçükgülmez (Equal). Visualization: Ali E. Kadak (Lead). Writing – review & editing: Ali E. Kadak (Lead). Funding acquisition: Ali E. Kadak (Equal), Aygül Küçükgülmez (Equal), Osman Gülnaz (Equal), Mehmet Çelik (Equal). Supervision: Osman Gülnaz (Equal), Mehmet Çelik (Equal). Data curation: Osman Gülnaz (Equal), Mehmet Çelik (Equal).
Data Availability Statement
The data are contained within the article, and the corresponding author can make more data available upon request.
Conflicts of Interest
The author declared no conflict of interest.


_and_after_(b)_dye_adsorption.jpeg)
_and_after_(b__c__d)_dye_adsorption.png)





_and_after_(b)_dye_adsorption.jpeg)
_and_after_(b__c__d)_dye_adsorption.png)