Introduction
The pearl oyster, Pinctada maxima, is known for producing large-sized nucleated pearls, among the most expensive worldwide. The species is naturally distributed in the central Indo-Pacific region from Myanmar to the Solomon Islands, including the Philippines, China, Australia, Papua New Guinea, Indonesia, Polynesia, Micronesia, and southern Japan.1 In China, P. maxima naturally exists along the coastal areas of southern provinces, such as Hainan, Guangxi, and Guangdong provinces. Researchers have studied the protocols for seed production and pearl culturing of P. maxima since the early 1970s, while pearl production in this species was experimentally successful in the 1980s. However, the P. maxima pearl production industry has developed slowly over the past decades due to over-fished wild populations and mass mortality at juvenile stages. Aiming to solve this problem, researchers improved the survival rates by developing rearing protocols for larvae, spat, and adults and introducing wild populations.1
Indoor farming is an alternative mode for P. maxima juvenile cultivation to improve survival rates, and sufficient food is crucial. Like many other bivalves, P. maxima filters water-suspended particles, such as microalgae, bacteria, organic debris, and microzooplankton. Studies on nutritional requirements and artificial feeds of bivalve mollusks are relatively rare compared to those on other aquatic animals.2 Microalgae provide major and highly variable nutritional value. Cell size, shape, and biochemical composition determine microalgae’s nutritive quality and utility as a food.3 The partial or total replacement of microalgal foods by an easily handled substitute with the same nutritional qualities has been attempted by hatcheries, such as microalgal concentrate,4 spray-dried microalgae,5 bacteria,6 yeasts,7 and so on.
A previous study suggested that the spray-dried microalgal diets (Isochrysis zhanjiangensis and Platymonas subcordiformis powder) can serve as substitutes for live microalga in P. maxima juvenile indoor farming under controlled conditions, assuring the survival rates as live microalgae.1 However, more research is needed to explore the effect of dietary manipulation on P. maxima juvenile growth and physiology, aiming to optimize healthy management under indoor farming mode.
In the present study, different microalgae-based diets were administered to P. maxima juveniles in an indoor hatchery farming condition. This study aimed to assess and compare the juveniles’ growth performance digestive and antioxidant capacity. To the best of the authors’ knowledge, this is the first effort to investigate the effects of live and spray-dried microalgae simultaneously on these oyster species under indoor farming conditions. The findings derived from this study would provide a practical approach to improving the health management of P. maxima juveniles in indoor farming conditions.
Materials and Methods
Experimental animals and management
The P. maxima juveniles were obtained from Sanya Maifeng Industrial Co., LTD (Hainan, China). Before the experiment, the juveniles were temporarily cultured for seven days in an aerated cement tank (29 oC, 30.0 psu, pH 8.20), fed daily with a mixture of Isochrysis zhanjiangensis, Platymonas subcordiformis and Chaetoceros muelleri. A daily 50% water exchange was conducted to guarantee optimal water quality for the well-being of P. maxima juveniles. The criteria for water quality maintenance included maintaining total ammonia-nitrogen levels < 0.02 mg/L and DO (dissolved oxygen) ≥ 7 mg/L.
The juveniles (2.12 ± 0.09 g in mean initial weight, 32.54 ± 0.43 mm in mean initial shell length, 29.97 ± 0.49 mm in mean initial shell height) were randomly assigned to experimental tanks. According to the different feeding strategies, triplicate tanks (1000 L) containing 300 juveniles were used for each rearing group.
Experimental design and management
A total of six experimental groups, namely D1, D2, D3, D4, D5, and D6 group, were set up. D1, D2, and D3 groups solely fed on live I. zhanjiangensis, P. subcordiformis and C. muelleri respectively, while D4, D5, and D6 groups solely fed on commercial spray-dried microalgae powder (the main component were I. zhanjiangensis, P. subcordiformis and C. muelleri powder respectively). Animals were fed every 12 hours. A total 50% water was replaced every day, and the experimental period lasted for 45 days. To control the final concentration of microalgae in the experimental tanks, the feeding quantity was determined by measuring the original concentration of the different microalgal diets precisely. Prior to daily feeding, 2 g of each spray-dried diet was suspended in 1000 mL of filtered seawater and pulsed in a commercial blender (JS39D-250, Supor, China). The liquefied mixture was then filtered through a 50-μm mesh to eliminate foam and larger particles and then filled up to 30 L with filtered seawater. This diluted suspension was then used in feeding. All diets were fed at a final of concentration of 150,000 cells / mL. The cell counts were carried out daily for each diet using a hemocytometer.
Live microalgae culture and commercial spray-dried microalgae powder sourcing
Microalgae stock cultures were obtained from the Microalgae Laboratories of Ocean College in Hainan University. These microalgae were cultured in 5 L and 45 L glass buckets and grown at 25.0 to 27.0 oC in Ningbo 3# nutrient medium. The photoperiod was 14:10 h (light/dark), and illumination was provided by the light fluorescent tubes (40-W). Filtered (0.45 mm) and UV-treated seawater (salinity, 31 psu) were used. Continuous aeration was provided to enhance growth and prevent the algae from settling. The microalgae for feeding were harvested during the exponential phase. SDIC Biotech Investment Co, Ltd (Beijing, China) provided the commercial spray-dried microalgae powder.
Chemical analysis for diets
The crude protein, ash, and lipid of all diets used in the experimental groups were measured for each group. Samples were dried at 80 oC to constant weight, and protein, ash and lipid were then determined. All measurements were performed in triplicate. The protocols for measuring were followed by Yang et al.2
Survival rate analysis
At the end of feeding trials, each group’s surviving pearl oysters P. maxima were counted to calculate the survival rate (SR). The SR was calculated following the equation: Survival rate = (the number of surviving individuals at the end of the experiment / the number of individuals at the beginning of the experiment) * 100%.
Biometric data collection and analysis
Shell biometric data such as shell length (SL), shell height (SH), and weight were collected for each group at the beginning and the end of the feeding trials. The SH and SL were measured using a vernier caliper (DL90150, Deli). The weight was obtained using an electronic balance (ME104E, Mettler Toledo). The following equations calculated the relative growth rate (RGR) and specific growth rate (SGR) for SL, SH, and weight. RGR = (X2 - X1) / X1 * 100, and SGR =100 * (Ln X2 – Ln X1) / t. Where “X1” and “X2” are the average of weight (or SL, or SH) at the beginning and the end of the experiment, respectively, and “t” is the experimental period in days.
Sample collection for biochemical assays
All experiments complied with the standards of the Guidance of the Care and Use of Laboratory Animals at Hainan University. At the end of the feeding experiments, the visceral mass of each pearl oyster was dissected and stored in liquid nitrogen for further analysis. Ten samples were collected from each replicate. The frozen tissues were homogenized on ice in 0.2 M (w/v) of ice-cold physiological saline, and homogenates were centrifuged at 13,000 g for 10 min at 2oC. The supernatant was separated for enzyme analysis in triplicate. The amylase (AMS, E.C.3.2.1.1), pepsin (PES, E.C.3.4.23.1), lipase (LPS, E.C.3.1.1.3), superoxide dismutase (SOD, E.C.1.15.1.1), acid phosphatase (ACP, E.C.3.1.3.2), and lysozyme (LZM, E.C. 3.2.1.17), were used as biochemical indicators. All enzyme activity was measured and detected with commercial reagent kits (Nanjing Jiancheng Bioengineering Institute, China). This procedure was carried out according to the manufacturer’s instructions.
Statistical analysis
All data are presented as mean ± standard error. For all statistical tests, P values <0.05 were considered to be significant. All statistical analyses were performed using DPS 14.5 software (Hangzhou Rui Feng Information Technology Co. Ltd., Hangzhou, China). All data were analyzed by one-way analysis of variance (ANOVA), followed by LSD test. Before analysis, data were tested for normality using Kolmogorov–Smirnov’s test and for homogeneity of variance using Cochran’s C test. Data that did not meet the ANOVA assumptions were log-transformed before analysis, and percentage data were arcsine square-root-transformed.
Results
Proximate compositions of the diets used in the feeding experiment
There were significant variations in the proximate compositions (% of dry weight) among different diets (P < 0.05, Table 1). The contents of crude protein in D1 (50.23 ± 1.05 %), D3 (49.62 ± 1.98 %), D4 (50.63 ± 2.32 %) and D6 (48.89 ± 2.42 %) diets, were significantly higher than that in D2 and D5 diets. The highest crude lipid content was observed in D4 (16.10 ± 2.03 %), followed by D1 without significant difference (P > 0.05). While the lowest lipid content (1.06 ± 1.41 %) was in D2 (P < 0.05). Similarly, the highest ash content was observed in D1, followed by D2 and D4 diets.
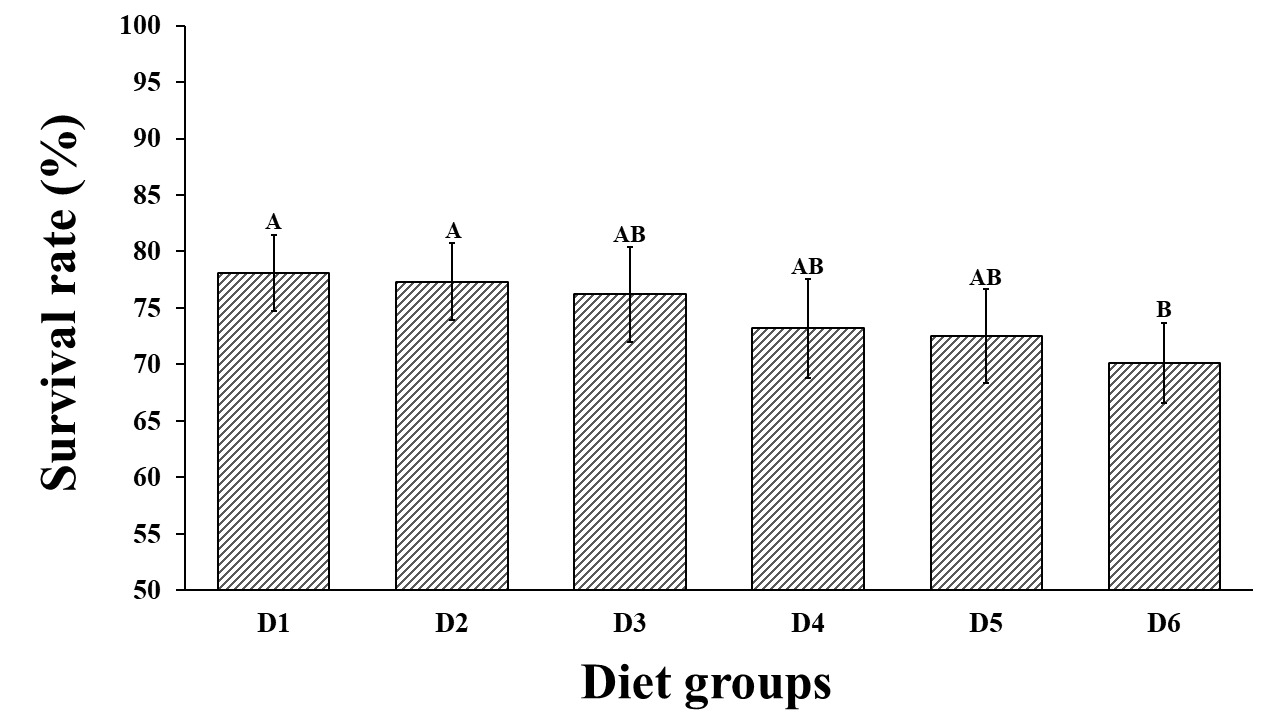
Survival rate of juveniles fed with the different diets
The survival rate differences were observed among the oyster groups fed different diets. Survival rates ranged from 70.13 ± 3.53% to 78.12 ± 3.37% (P < 0.05, Figure 1). The lowest survival rate was found in the oyster group fed with D6 (C. muelleri powder, group-D6), while the highest survival rate was exhibited by the group fed with D1 (live I. zhanjiangensis). Significant differences were only observed between group-D6 and group-D1, as well as group-D2 (77.34 ± 3.45%, group-D1).
Growth performances of pearl oyster juveniles
The biometric parameters (weight, SL, and SH) of P. maxima juveniles registered at the commencement and the end of the feeding trials, with their corresponding RGR and SRG, are shown in Table 2. Group D1 exhibited the highest biometric values registered at the end of the experiment. In this group, the weight, SL, and SH were 2.63 ± 0.17 g, 37.92 ± 0.57 mm and 35.37 ± 0.68 mm respectively. Group D3 exhibited the second-highest performance. The final weight in groups D2, D4, D5, and D6 were similar and ranged from 2.26 g to 2.32 g (P > 0.05), significantly lower than that in group D1 (P < 0.05). Similarly, the final SH in groups D2, D4, D5, and D6 were similar, ranging from 31.28 mm to 33.54 mm, without significant difference (P > 0.05), but significantly lower than that in group D1 and group D3 (34.81 ± 0.58 mm, P < 0.05). Furthermore, there were significant variations in the final SL among diet groups. The SL in groups D3 and D4 (36.50 ± 0.41 mm and 36.25 ± 0.57 mm, respectively) was significantly higher than that in groups D2 and D5 (P < 0.05) but significantly higher than that in group D1 (P < 0.05).
The group-D1 exhibited the highest calculated values for RGR and SGR concerning weight (24.00 ± 2.18%, 0.72 ± 0.06% / d), SL (16.62 ± 0.04%, 0.51 ± 0.01% / d), and SH (17.92 ± 0.10%,0.55 ± 0.02% / d). These values were significantly higher than those observed in the other experimental groups (P < 0.05). Conversely, group D5 exhibited the lowest growth rates among all the experimental groups.
Digestive enzyme activity
Different diets significantly influenced P. maxima juveniles’ digestive enzyme activity (P < 0.05, Figure 2). Group D1 exhibited the highest amylase, pepsin, and lipase activity, with values of 1.37 ± 0.10 U/mg protein, 5.22 ± 0.14 U/mg protein, and 5.22 ± 0.14 U/mg protein respectively. These values were significantly higher than those observed in the D2, and D5 groups (P < 0.05). Conversely, the lowest values were observed in the D5 group, with amylase, pepsin and lipase activities of 1.01 ± 0.13 U/mg protein, 4.59 ± 0.18 U/mg protein, 11.95 ± 1.29 U/mg protein, respectively.
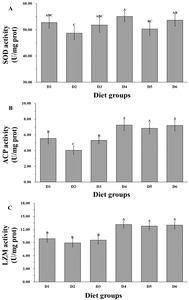
Antioxidant and immune enzyme activity
Antioxidant- and immune-related enzyme activity of P. maxima juveniles from different diet groups differed significantly (P < 0.05, Figure 3). The group-D4 exhibited the highest activity levels of SOD, ACP, and LZM, with values of 55.10 ± 2.03 U/mg protein, 7.25 ± 0.70 U/mg protein, and 13.13 ± 0.80 U/mg protein, respectively. Following closely, group D6 exhibited 53.63 ± 2.19 U/mg protein, 7.19 ± 0.71 U/mg protein, and 13.01 ± 0.90 U/mg protein, respectively (P > 0.05). Conversely, the lowest SOD, ACP, and LZM activity were observed in group-D2, with values of 48.73 ± 2.57 U/mg protein, 4.05 ± 0.55 U/mg protein, 8.95 ± 0.95 U/mg protein, respectively. The values were significantly lower than those observed in the D4 and D6 groups (P < 0.05).
Discussion
The physicochemical parameters of the seawater during the experiments were DO 7-8.2 mg/L, pH 8.10 to 8.20, total ammonia-nitrogen 0.00 to 0.01 mg/L, and the nitrite-nitrogen concentration cannot be detected. These values were considered homogeneous and normal. The stable and suitable water quality can promise the P. maxima juvenile were living in good condition. The survival rate exceeded 70% in all the experimental groups. Notably, the survival rates of juveniles fed with the commercial spray-dried microalgae powder (except C. muelleri powder) were not significantly different from those fed on live microalgae. Similarly, it has been reported that the mortality in the green-lipped mussel Perna canaliculus spat fed on MySpat diet was equivalent to those fed on microalgae in the dietary control.8
Growth performance is the main component influencing economic benefits in commercial aquaculture facilities, and growth is an ongoing process subjected to many positive and negative influences.9 Nutrition is one of the key factors for successful culturing, and dietary manipulation is a useful approach to improve the production and health of farmed aquatic livestock. Pearl oysters filter water-suspended particles, and microalgae provide a dominant and highly variable nutritional value.2 Microalgae feature variable nutritional values and the major organic components comprise protein (30% – 40% of total dry weight), lipid (10%–20% of total dry weight), and carbohydrate (5%–15% of total dry weight).10 The proximate compositions of the diet used in the present study, including live and spray-dried microalgae, were consistent with the aforementioned proportions. However, there were significant variations in the proximate compositions among different diet groups, regarding the live and spray-dried I. zhanjiangensis containing the higher crude protein and lipid, followed by C. muelleri.
In aquaculture farming activities, absolute growth rate (AGR), relative growth rate (RGR), and specific growth rate (SGR) are parameters often used for growth performance evaluation. The RGR is a useful metric for comparing the growth of individuals of the same initial size when subjected to different treatments, as it considers the initial size of the individuals. It is useful to measure the percentage growth per day when conducting nutrition-related experiments with groups of aquatic livestock over a short period, especially the small one.11,12 At the commencement of the experiment, P. maxima juveniles had a uniform and small initial size. The calculated RGR and SGR values significantly differed among the experimental groups, with the better growth performance (weight, SL, and SH) exhibited by juveniles fed on live I. zhanjiangensis, and live C. muelleri. Conversely, the spray-dried microalgae powder was not as good as the live diets. These results indicated that the diets affected the growth of pearl oyster juveniles.
The differences in growth performance also could be related to the role of digestive enzymes and subsequent nutrient enhancement. Feed is essential for successful and profitable aquaculture activity, especially the nutritional value of diets that is usually related to the host’s digestive capability and nutrient utilization.13 Digestive enzymes, such as amylase, pepsin, and lipase, are involved in the digestive processes of aquatic animals, and always serve as important indicators of the physiological status, digestive processes and nutritional condition of living organisms.1,14 In the present study, the differences in digestive enzymes activity among the different diet groups, were in concordance to the difference in growth performance. This is consistent with the results reported in a sea bass study.14 Furthermore, aquatic animal growth performance may decline to some extent due to amino acid distortion,15 thus the specific mechanism for P. maxima still needs further study.
Reactive oxygen species (ROS) normally increase when animals are subjected to stress, resulting in oxidative stress. The digestive system in most marine animals is easy to be affected by ROS, resulting in a negative influence on normal physiological metabolism functions.16 To cope with it, physiological responses normally happen, especially antioxidant defense systems.17 Antioxidant capacity includes enzymatic and non-enzymatic antioxidant activities. Antioxidant enzymes participate in primary antioxidant protection against free radicals in organisms, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and so on. The SOD acts as the initial barrier against oxidative stress, by neutralizing the superoxide anion. Subsequently, CAT breaks down the hydrogen peroxide produced from the SOD reaction, transforming it into water and oxygen.18 Available reports evaluated the effects of nutritional factors on the antioxidant capacity of different aquatic animals, such as golden pompano Trachinotus ovatus,19 pacific white shrimp Litopenaeus vannamei,20 grass carp Ctenopharyngodon idella,21 sea cucumber Apostichopus japonicus,22 pearl oyster Pinctada fucata martensii.2 Furthermore, scientific evidence indicated that dietary nutrients can stimulate the immune systems of aquatic animals.23 Shellfish species have defensive mechanisms that mainly rely on innate immune responses, because they lack an acquired immune system.24,25 The acid phosphatase (ACP) is involved in various metabolic processes, such as detoxification, metabolism and biosynthesis of macromolecules, for various essential functions in living organisms.26 The lysozyme (LZM) is released by leucocytes, and can be used as a marker for the innate immune system in aquatic animals.27 These compounds are both important in marine invertebrates, and may participate in nonspecific immune actions.28 The results reported in the present study indicated that diets affected the antioxidant capacity of P. maxima juvenile, where the ACP and LZM values in juveniles fed with spray-dried microalgae powder groups were higher than those fed with live microalgae. With all this evidence, including the results of the aforementioned reports and this study, it is clear that diets affected the antioxidant capacity of pearl oysters.
In summary, this study is the first to investigate live microalgae and algae powder effects on growth performance, digest and antioxidant capacity of pearl oyster Pinctada maxima juvenile over 45 days indoor farming. The survival, growth and productive performances, the activity of digestive, antioxidant and immune enzymes were used as indicators for comparative profiling. The results showed that the juvenile survival rates fed with spray-dried microalgae powder (except Chaetoceros muelleri powder) were without a significant difference to those fed on live microalgae (Isochrysis zhanjiangensis, Platymonas subcordiformis and Chaetoceros muelleri), even though the growth performance of juveniles fed with spray-dried microalgae powder was not as good as the live one. Furthermore, we found that the digestive enzymatic activities were consistent with growth performance, and diets evidently affected the antioxidant capacity. The results in the present study suggested that the spray-dried I. zhanjiangensis powder can serve as a substitute of live microalga in P. maxima juvenile indoor farming, and recommended in the present study under controlled conditions. These findings would provide basic data to improve health management for P. maxima juveniles at indoor farming conditions.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2022YFD2401303), pearl oyster Pinctada maxima seeding project from Lingao Forest Farm of Lingao County (ZJ-Qiong-2023-227-2), and the Academician Innovation center of Hainan Province, P. R. China.
Declaration of competing interest
The authors declare no conflict of interest.
Authors’ Contribution per CRediT
Data curation: Yi Li (Equal), Hebert Ely Vasquez (Equal). Formal Analysis: Yi Li (Equal), Hebert Ely Vasquez (Equal). Investigation: Yi Li (Equal), Ze Yin (Equal), Yu Chen (Equal). Resources: Hebert Ely Vasquez (Equal), Xing Zheng (Equal), Zhifeng Gu (Equal). Software: Hebert Ely Vasquez (Equal), Xing Zheng (Equal), Zhifeng Gu (Equal). Validation: Hebert Ely Vasquez (Equal), Feng Yu (Equal), Xing Zheng (Equal), Zhifeng Gu (Equal). Writing – review & editing: Hebert Ely Vasquez (Equal), Xing Zheng (Equal), Zhifeng Gu (Equal). Visualization: Ze Yin (Equal), Meng Zhang (Equal), Shuaiqin Lan (Equal). Writing – original draft: Yu Chen (Equal), Jing Mao (Equal), Lingfeng Wang (Equal), Shangkun Wei (Equal). Methodology: Jing Mao (Equal), Lingfeng Wang (Equal), Meng Zhang (Equal), Shuaiqin Lan (Equal), Shangkun Wei (Equal). Supervision: Feng Yu (Equal), Zhifeng Gu (Equal). Conceptualization: Xing Zheng (Lead). Funding acquisition: Xing Zheng (Equal), Zhifeng Gu (Equal). Project administration: Xing Zheng (Lead).