Introduction
Cadmium (Cd) is known to be a carcinogenic, teratogenic, and mutagenic heavy metal because it is considered as the second most dangerous metal in the environment and can have an extremely toxic effect on living organisms, and is also recognized as one of the main environmental pollutants in aquatic ecosystems.1,2 In recent years, with the accelerated development of the industrialization process, a large amount of wastewater containing Cd is discharged at random, which makes Cd to enter the aquatic environment from industrial processes, chemical agricultural manuring, and mining activities.3 This phenomenon seriously threatens aquatic organisms’ survival and safety, resulting in Cd accumulation in aquatic organisms such as fish, shrimp, crabs, and shellfish.4 Long-term Cd exposure may lead to the results of some physiological disturbances to the brain, liver, gill, gonad, kidney and other organs of aquatic animals,5 leading to adverse impacts such as skeletal developmental anomalies, disorders in metabolic systems and reproductive endocrine, reduced activity of oxidative metabolic enzymes, immunity suppression and so on.6–8 Ultimately, the ecotoxicological effects of Cd contamination led to a dysregulated physiological activity and even death of individual organism.9,10 As a result, the pollution of Cd can lead to huge economic losses to fishery production, causing potential safety risks of aquatic product quality through the food chains and affecting human health.
Largemouth bass (Micropterus salmoides), also known as California perch, is a carnivore fish belonging to Heliocephalidae and the genus Lateolabrax. Largemouth bass greatly benefits consumers due to its delicious flesh, lack of intramuscular bones, rich nutrition, and abundance of essential amino acids and unsaturated fatty acids that the human body needs. Additionally, largemouth bass grows rapidly and has strong disease resistance, which has become one of the important freshwater aquaculture fish species in China.11,12 However, with the frequent human industrial and agricultural activities, the pollution of water ecology has been further aggravated. Therefore, our study focuses on the effects of 28 days of exposure to different Cd concentrations on gill and liver damage, antioxidant capacity, serum biochemical indices, and gene expression in the liver of juvenile largemouth bass. Results reveal the potential mechanism of Cd toxicity in juvenile largemouth bass and provide valuable clues for heavy metal poisoning in aquatic organisms.
Materials and methods
Ethics Statement
All animal protocols and procedures were performed according to the Administration of Affairs Concerning Experimental Animals in China (Ministry of Science and Technology, revised in June 2004). The Henan University of Science and Technology Institutional Animal Care and Use Committee approved the experiments in this study.
Experimental fish
The experimental fish was purchased from a commercial breeding company in Jili District, Luoyang, Henan Province, with the same batch of fry (average weight: 7.04 ± 0.25g, wet weight; standard length: 7± 0.25 cm). Before the experiment began, fish were temporarily raised in the indoor recirculating aquaculture system of Henan University of Science and Technology for a 14-day acclimatization. Fish were fed with a commercial fish diet (Zhuhai HaiLong Co. Ltd., Guangdong, China).
Exposure experiments
The water used in the experiment is tap water with aeration for 3 days. Each group of fish was exposed to Cd concentration of LC50 and carried out for 28 days as follows: Group H (high concentration group): 0.25 mg/L of water (100% of the LC50); Group M (medium concentration group): 0.01 mg/L of water (50% of the LC50); group L (low concentration group): 0.05 mg/L of water (12.5% of the LC50) and group C (control): containing 0.0% Cd. Three hundred and sixty fish were divided into four experimental groups with triplicates (30 fish per tank). Each aquarium contains 100 L of aerated tap water. During the experiment, water quality was maintained with pH: 7.5±0.2, temperature: 24±2 °C, dissolved oxygen>5.0 mg/L, and nitrite<0.5 mg/L.
Sample collection
After the end of the exposure experiment, all experimental fish were starved for 24 hours before sampling. Next, fish were anesthetized using MS-222 (120 mg/L). Gills and livers of four fish from the same tank were sampled and snap-frozen in liquid nitrogen before storage at -80 °C until analyzed. Three fish (per tank) were randomly selected for gill and liver histological samples. Six fish (per tank) were randomly sampled, and their blood was collected by heart puncture, and then serum was obtained by centrifugation (3500 rpm) at 4℃ for 10 min and stored at -80℃ until analyzed. Similarly, liver tissues from six fish (per tank) were collected and stored at -80℃ for further molecular analysis.
Observation of the tissue structure
The gill and liver were fixed in 10% neutral formalin buffer solution and then sent to Wuhan Servicebio Biotechnology Company (Wuhan, China) for paraffin section preparation. Briefly, the samples were trimmed with a scalpel and dehydrated in ethanol. Subsequently, the samples were equilibrated in xylene, embedded in wax for 2 h at 60℃, and then sectioned to 5-7 µm thickness using a rotary Nikon Eclipse Ci-L (Nikon, Tokyo, Japan). Finally, the samples were stained with hematoxylin-eosin (H&E) and then photographed for observation.
Antioxidant enzyme activity
The total antioxidant capacity (T-AOC), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) activity and malondialdehyde (MDA) content were measured by commercial assay kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the instructions.
Serum biochemistry parameters assay
Serum biochemical indices were measured using an automatic biochemical analyzer (AU-5800, Beckman) at the New Area Hospital Inspection Center of the First Affiliated Hospital of Henan University of Science and Technology (Luoyang, China). The measured indicators include alanine transaminase (ALT), aspartate transaminase (AST), total protein (TP), urea nitrogen (UN), glucose (GLU), triglyceride (TG) and total cholesterol (TCHO).
Real-time PCR analysis.
Total RNA was extracted from the liver tissue of juvenile largemouth bass using TRIzol reagent (Dalian, Takara) according to the method recommended by the instructions. Total RNA quantity and quality were evaluated using a Nano DropND-2000 spectrophotometer (Nano-Drop Technologies, Wilmington, USA). Total RNA integrity was identified by 1.2% agarose gel electrophoresis. 1μg total RNA was then reverse transcribed with a PrimeScript RT Reagent Kit With gDNA Eraser (Dalian, Takara). The obtained cDNA was then stored at -20℃ for future use. Real-time PCR was performed using the SYBR Premix Ex TaqTM kit (Dalian, Takara) using an MJ Chromo4 Real-Time cycler (Bio-Rad, CA, USA). The reaction volume was a total of 25 μL. The PCR reaction program was 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s. Each sample was run in duplicate, and β-actin was chosen as internal reference gene. Amplification results were calculated using the 2−ΔΔCt method.13 All primers were listed in Table 1.
Statistical analysis
Excel 2016 software was used to process the obtained experimental data, which were then subjected to statistical analysis using SPSS Statistics 26.0. Differences among exposure groups were tested via One-way ANOVA and Dunnett’s T3. All values are represented as means ±SE. P<0.05 was considered as significantly different.
Results
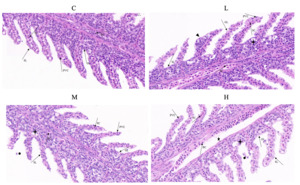
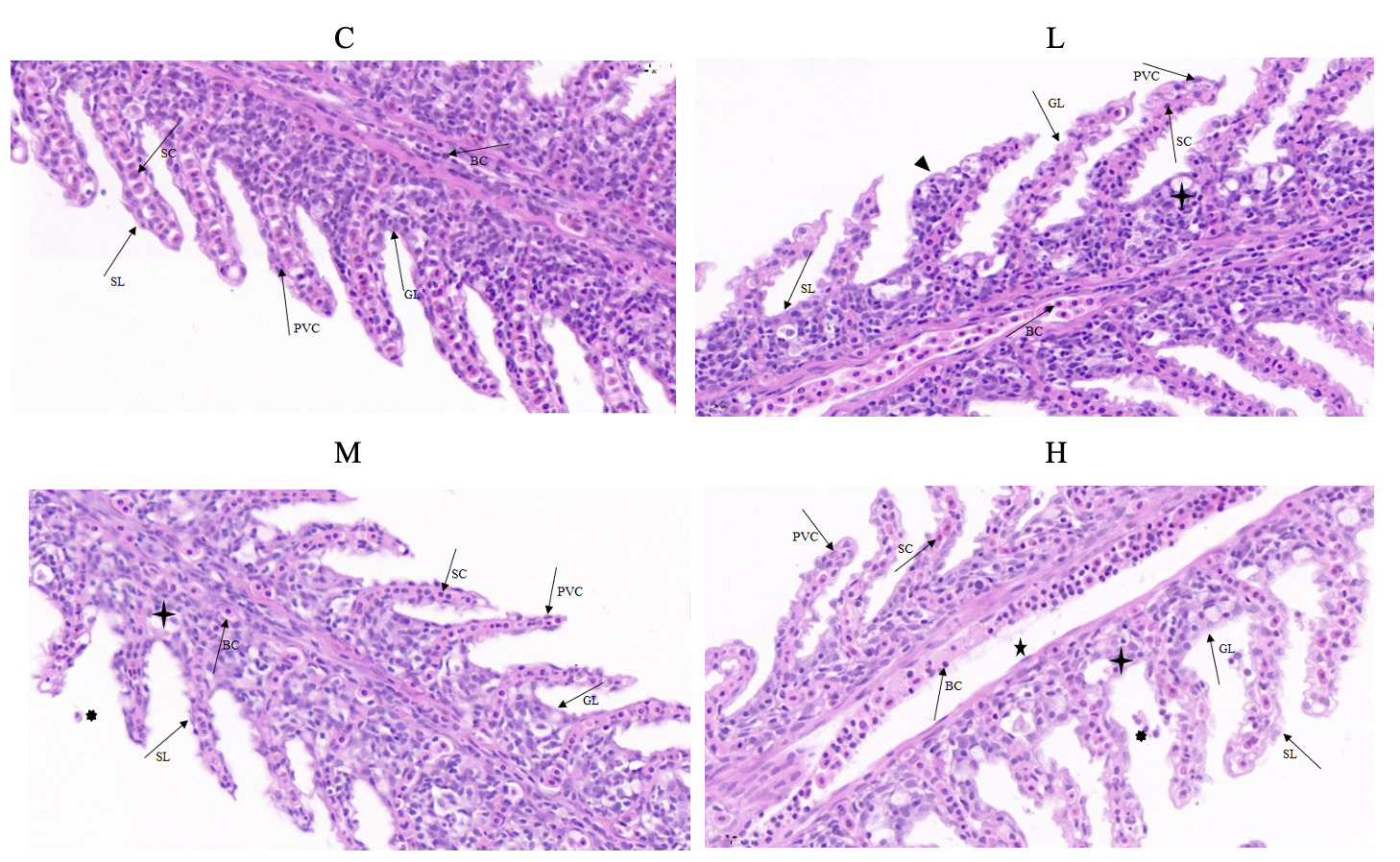
Effects of Cd exposure in gill histopathology
After 28 days of Cd exposure, the alterations in the gill histopathology are shown in Figure 1. In group C, the gill histological structure revealed an integrity in which cells were closely arranged and cell boundaries were clear. In group L, partial pavement cells (PVCS) of secondary gill lamella appeared swollen, and the cells began to show slight vacuolation. In group M, the PVCS swelling in secondary gill lamella was further expanded, a few cells fell off, and some cells exhibited mild vacuolation. In group H, the PVCS was further seriously swollen, the vacuolation rate significantly increased, and cell detachment was accelerated. The structural arrangement of the columnar cells and PVCS in the gill were changed and disordered.
Effects of Cd exposure in liver histopathology
After 28 days of Cd exposure, the liver morphological structure of juvenile largemouth bass is shown in Figure 2. In group C, the liver cells exhibited an entire roundish polygonal structure containing an apparent spherical nucleus. The liver cell structure in group L remained relatively entire, with clear cell boundaries. A small number of liver cells were slightly altered in shape structure. In group M, the liver cells’ structure has partial incompleteness, in which the cell shapes have changed. The cell boundaries are indistinct and appear to be vacuolization and fusion phenomena. In group H, the structure of liver cells was damaged aggregately in many places, accompanied by nuclear shrinkage and cellular necrosis.
Effects of Cd exposure on antioxidant enzyme activities in the gill
As shown in Table 2, in comparison to group C, the SOD activity in the gill of juvenile largemouth bass was significantly higher in group L and group M during 28 days of the Cd exposure (P<0.05); whereas the SOD activity in group H was significantly decreased (P<0.05). The results indicated that with increasing concentration of Cd exposure, the SOD activity was gradually elevated, high concentration of Cd exposure can inhibit the SOD activity. The content of MDA increased significantly (P <0.05), but there was no significant difference between group L and group M (P>0.05). The GPx activity did not change significantly in group L and group M (P>0.05), while GPx activity was significantly lower in group H (P<005). The CAT activity was significantly increased in group L and group M (P<0.05), but its activity in group H did not change significantly (P>0.05). T-AOC showed a decreasing trend, but no significant difference existed between all the groups (P<0.05).
Effect of Cd exposure on antioxidant enzyme activities in the liver
As shown in Table 3, compared to the control group C, the SOD activity in the liver of juvenile largemouth bass was significantly increased in group L and group M after 28 days of the Cd exposure but significantly decreased in group H (P<0.05). MDA content was significantly increased among all the groups (P<0.05). The GPx activity in group L and group M slightly increased but had no significant change (P>0.05); however, the GPx activity in group H was significantly decreased (P<0.05). The CAT activity increased with the increase of Cd concentration, but there was no significant change in group L and group C (P>0.05); however, the CAT activity in group M and group H was significantly increased (P<0.05). T-AOC showed a decreasing trend, but there was no significant change among all groups (p>0.05).
Effects of Cd exposure on serum biochemical parameters
The blood biochemical parameters of juvenile largemouth bass after 28 days of exposure to different Cd concentrations are shown in Table 4. By comparison with group C, the ALT and AST activities were significantly increased in group M and group H (P<0.05), but had no significant difference in group L (P>0.05); the contents of GLU, TC, and TG did not change significantly in the group L and group M (P>0.05), but increased significantly in group H (P<0.05); The content of ALB and TP increased slightly in the group L compared with the group C, and decreased significantly in the group M and group H (P<0.05). Still, there between these two groups (p> 0.05).
Effects of Cd exposure in the liver-related gene expression
The effects of gene expression after 28 days of exposure to different Cd concentrations in the livers of juvenile largemouth bass are shown in Figure 3. The expression level of HSP70 in group M was significantly higher than that of group C and the other two groups (P<0.05); the expression level of HSP70 in group H was not significantly different from group C and group L (P>0.05). The expression levels of HSP90 and HSP70 showed the same trend, but the expression level of HSP90 was slightly lower than that of HSP70. With increased Cd concentration, MT expression level was significantly upregulated in group H (P>0.05). The expression level of CYP 1A was significantly higher and reached a maximum value in group L (P>0.05), and decreased gradually in group M and group H.
Discussion
Previous studies indicated that when fish are exposed to Cd-contaminated water for a long time, Cd accumulates continuously in organs such as the gill, liver, and kidney, exceeding the fish body detoxification and excretion of Cd, resulting in organs or tissues pathologically damaged. Subsequently, the biotoxicity of Cd occurs, which can cause pathological damage to organs or tissues, causing cell apoptosis and necrosis.14,15 The accumulated toxicity of Cd can be observed in the gill and liver of fish because the liver is one of the vital detoxification organs in fish in which foreign pollutants are bio transformed and metabolized.16 The gill is an essential respiratory organ of fish and serves as a site for acid-base balance and eliminating nitrogenous wastes from the body. Pathological changes of the gill can reduce the efficiency of gill gas exchange, cause an imbalance of ions in the body, and finally lead to feeding difficulties or individual death of fish.17 For example, after exposing spear-tailed goby (Synechogobius hasta) to water containing 0.29 mg/L Cd for 15 days, damages such as aneurysms and cellular hyperplasia were observed in the gill.18 A study from Thophon et al. reported that when Lates calcarifer was exposed to water containing 20.12 mg/L Cd for 72 h, apparent pathological damage such as cell proliferation, epithelial cell detachment, and necrosis was observed in the gill.19 Low Cd concentration did not cause gill damage in fish. Still, when Cd concentration exceeds the gill’s tolerance level, it could cause histological damage, resulting in cellular inflammation, apoptosis, necrosis, mitochondrial vacuolization, and swelling.20 This is consistent with our present results. High Cd concentration caused lysis of gill filament epithelial cells, with many vacuoles and even detachment. As an important detoxification organ of fish, the liver mainly protects the tissues from damage where the liver can convert heavy metals into less toxic substances to be excreted from the organisms. When accumulated Cd concentrations in the liver exceed its detoxification capacity, it can affect tissue integrity and functional liver damage to varying degrees.21 Accumulation of Cd and cell damage degree were positively correlated with time of exposure and concentration of Cd.22 Usha et al. studied the toxicity of Cd on tilapia and found that after Cd exposure, liver cells exhibited hepatocyte hypertrophy, fatty degeneration and appeared tissues damage including a large number of vacuoles and liver vascular congestion.23 Similar observations were found in Dicentrarchus labrax and Lates calcarifer in which long-term exposure of fish to water containing Cd led to pathological changes such as cytoplasmic granulation, cellular edema and vacuolization, lipid droplet accumulation, and increased glycogen granules in the liver.19,24 Our studies showed that the liver of juvenile largemouth bass could maintain functional detoxification under low concentrations of Cd exposure. However, after Cd exposure for a long time and high concentration, the membrane structure of the liver cells was changed, vacuolated seriously, and the shape of the cells changed. This finding implied that the high concentration of Cd exposure had noticeable toxic effects on the liver, which was consistent with previous studies.
SOD, CAT, and GPx are key enzymes in the antioxidant system of the organism, which play a role in scavenging superoxide radicals in the body and protecting cells from oxidative damage, thus achieving detoxification.25,26 Our results showed that the activity of SOD in the gill and liver of juvenile largemouth bass increased first and then decreased with the increase of Cd concentrations. Low Cd concentration induced an increase in SOD activity; however, with Cd concentrations increasing, the activity of SOD decreases, which may be due to the oxidative substances produced in the body exceeding its defense capacity, resulting in the cell destruction, dysfunction and a decrease of the activity; or it may be an adaptive protection mechanism evolved by the body in response to heavy metal poisoning.27 Similar to our results, the activity GPx of the gill and liver in group L and group M of Cd exposure was induced to scour free radicals. Still, the activity of GPx was inhibited in group H. However, it is noteworthy that the activity of CAT was significantly increased in the gill of group L, while no change was observed in the liver, except CAT activity reached a maximum in group H. This reason may be related to the difference in Cd accumulation in the different types of fish tissues. For example, the amount of Cd accumulation in the liver is usually several times higher than that in the gill and other tissues, and the high concentration of Cd accumulation causes a large amount of free radical production in the liver.28 MDA is a product of lipid peroxidation, a sign of oxidative damage that can indirectly reflect the degree of cell damage.29 In our present study, the MDA content in the gills and livers of juvenile largemouth bass showed a significant increase in Cd exposure groups compared to group C, indicating Cd-induced lipid oxidation in tissue cells. Similarly, previous studies have reported that Cd exposure increases MDA contents in fish such as Dicentrarchus labrax,30 Channa punctata,31 and Paraiichthys olivaceus.32 T-AOC is a comprehensive indicator of the antioxidant function in the organism, which directly reflects the ability of aquatic animals to resist stress. Our study showed that serum T-AOC tended to decrease after Cd exposure, although there are no differences among all groups; on the other hand, indicating that Cd has an oxidative damage effect on the organism.
Fish serum indicators are affected by the condition of the organism and environmental factors, which can reflect the health status and nutritional level of fish in which lipid and blood glucose levels are closely related to the metabolism and nutritional level of the organism, and the dynamic balance of these substances is very important for the health of the organism.33 Our results showed that GLU content was significantly increased in the group H, suggesting that juvenile largemouth bass themselves require large amounts of glycogen to be produced as energy for detoxification when exposed to Cd water, thus leading to increased GLU levels in serum. The contents of TG and TC showed an increasing trend in line with GLU and reached a maximum in the group H. Result indicates that the metabolism of these substances is also affected by Cd, and further studies are needed to be done in the future. ALT and AST are the most important transaminases involved in amino acid metabolism in liver and are often used as important indicators for the diagnosis of fish diseases.34 Under normal conditions, only a small number of transaminases are released into the blood from the animal cells, so the serum transaminase activity is very low. When the tissue damages, a large number of transaminases from cells is released into the blood, resulting in some significant changes in the enzyme activities. Kim et al. reported that Cd exposure could increase the activities of TG, TC, AST, and ALT in the hepatopancreas of Danio rerio, indicating that the hepatopancreas is damaged.35 In the present study, the activities of ALT and AST were significantly increased in group M and group H, indicating that the liver function may be impaired was caused. The elevated indicator is believed to be associated with liver injury in fish. This result agrees with the previous studies by Demerdash et al.36 and Oner et al.37 We speculated that ALB and TP might show a certain degree of self-adaptability in low concentration of Cd exposure with slight increase activities compared to group C; reversely, protein toxicity was only observed in the group M and the group H. The reasons for these results may be related to the protection of metallothionein (MT) containing sulfhydryl in the organism.38
Heat shock proteins are often used as biomarkers. When cells are exposed to environmental stress, their initial response is to over-express and synthesize a large amounts of heat shock proteins.39,40 Giri et al. reported that the expression level of HSP70 was significantly upregulated in the liver of Labeo rohita when fish was exposed to water with Cd 0.65 mg/L concentration.41 Similar observations were made by Ali et al., who found that the expression level of HSP70 in the liver of Cyprinus carpio was significantly upregulated with increasing of Cd exposure concentration and down-regulated expression in group H.42,43 This is consistent with our findings that the expression level of HSP70 increased first and then decreased in the liver of juvenile largemouth bass. In addition, the expression level of HSP90 was also significantly induced in group M after 28 days of exposure to different Cd concentrations. This is a similar result to the findings of Danio rerio and Xenopus laevis.44,45 It is speculated that the up-regulated expression level of HSP90 may result from HSP90 protein synthesis, which assists the denatured protein to repair itself and protect the organism when it appears to have heavy metal poisoning.46,47 The MT has been widely used as a biomarker for heavy metal treatment. Its protein structure contains a large amount of sulfhydryl, which can be combined with Cd and excrete heavy metal through a series of transport processes to reduce their toxic effects on the organism, so as to perform detoxification.48 In this study, the expression level of MT in liver of juvenile largemouth bass increased with the increase of Cd exposure concentration indicating that the liver of fish can remove Cd accumulation by increasing the protein expression level of MT, which is a physiological response to resist Cd stress. This is consistent with the reports that the upregulated expression level of MT can promote the expression of its own protein in various tissues such as the liver, gill, muscles, and kidneys from Mytilus galloprovincialis, Danio rerio, red snapper and Tilapia in which these tissues response to the different concentrations of Cd exposure.49–53 Cyp1A is also used as an early warning signal for pollutant monitoring, and its activity is affected by drugs and environmental pollutants.54,55 In the present study, the expression level of Cyp1A in the liver in group L was higher than that of group M and group H. This result is consistent with the trend of low-concentration induction and high-concentration inhibition of exogenous pollutants on the toxicity of aquatic organisms.
Conclusion
At the exposure of medium and high concentrations of Cd, histopathological damage and oxidative stress occurred in the gill and liver of juvenile largemouth bass. Exposure to the low and medium Cd concentrations can induce a stress compensation effect, leading to increased antioxidant enzyme activities in the gill and liver. In contrast, exposure to high Cd concentrations can inhibit the antioxidant enzyme activities in the gill and liver, thereby reducing the antioxidant capacity of cells. Meanwhile, low Cd concentration exposure had no significant effects on serum biochemical indicators. The expression level of HSP70, HSP90, MT, and Cyp1A in the liver of juvenile largemouth bass were influenced after exposure to different Cd concentrations. However, the specific mechanism of its impact still requires further in-depth research.
Acknowledgment
The present study was supported by the Program for Innovative Research Team (in Science and Technology) at the University of Henan Province(24IRTSTHN033)and the Key Research Project of Higher Education Institutions of Henan Province (23A240003).
Authors’ Contribution
Conceptualization: Hui Liu (Equal). Methodology: Hui Liu (Supporting), Yang Wang (Supporting). Formal Analysis: Hui Liu (Supporting). Writing – review & editing: Ke Fan (Supporting), Yuanyi Liu (Equal). Resources: Yuanyi Liu (Equal), Yumei Liu (Equal). Investigation: Yang Wang (Supporting). Funding acquisition: Yumei Liu (Supporting). Writing – original draft: Yong Huang (Lead). Supervision: Yong Huang (Lead).