Introduction
Snakehead (Channa sp.) is a carnivorous, air-breathing, freshwater fish species that belongs to the Channidae family and is distributed in freshwater areas of Southeast Asia.1 This fish species has high nutrition and economic value due to its beneficial effect on human health, high market demand, and resistance to low dissolved oxygen levels and turbidity. It is especially easy to raise at high density in different systems such as earthen ponds, net cages placed in ponds, or cement tanks.2 Snakehead has long been commercially cultured in many countries such as the Philippines, Cambodia, and Vietnam because it has nice tasty meat and good market value.3 Therefore, snakehead has become a promising candidate for freshwater aquaculture, a target species of intensive farming in South-East Asia.
In Vietnam, snakehead culture has rapidly developed in some provinces of the Mekong Delta, especially in the Dong Thap and An Giang provinces.4 They are considered to be one of the most common food fish and are a favorite among consumers due to their high taste and qualities.3 Snakehead farming is initially recognized as bringing economic efficiency and stable income for local households. However, snakehead fish farming costs are still so high due to the reliance on commercial feed or unstable and run-out sources of trash fish collected in nature. Among the costs of the snakehead culture, feeding represents the largest portion (accounting for 50-60% of production cost) of the economic balance; thus, the economic success of the fish production sector is mainly linked to the use of low-cost feed.5 Therefore, the primary goal of aquaculture nutritionists, as well as fish farmers is to reduce production costs, especially feeding expenses.6 On the other hand, consumers prefer to use organic aquaculture products instead of animals raised on commercial feed. Thus, finding an alternative natural feed that can be used as full or partial inclusion in the diets of snakehead needs to be studied.
Presently, the use of insects as direct feed or feed ingredient for aquatic animals has been widely studied. The exploitation of insects as feed ingredients is not in direct competition with food production,6 limiting the environmental impact of the production system, and contributing to a circular economy and a “zero waste” society.7 Among the insect species used as direct feed or protein source for aquaculture feed, Black soldier fly larvae (Hermetia illucens) is the most interesting source for its sustainability related to its capacity to convert organic waste material into biomass containing proteins (40–55%),8 fat (30–35%) with fatty acids of nutritional interest and ash (11–15%) with high mineral concentrations and a high Ca/P ratio,9 contributing to the food chain and reducing pollution.10 Moreover, Hermetia illucens is a harmless insect,8 with a short life cycle (40 – 45 days) and the possibility of biomass culture under small-scale farms.10 These advantageous characteristics prove BSFL to be an excellent feed candidate for aquaculture.
In aquaculture, Black soldier fly larvae (BSFL) have been studied and used as a direct feed or a partial replacement of fishmeal in the diets of Atlantic salmon,7 tilapia,11 carp,12 and seabass.13 A total replacement of fish meal with BSFL meal in the diets of sea-water Atlantic salmon was possible without negative effects on growth performance, feed utilization, nutrient digestibility, liver traits, or the sensory qualities of the fillet.7 Aisyah et al.11 documented that incorporating BSFL into a commercial diet for red hybrid tilapia fingerlings increased crude protein and fat composition, providing an alternative protein and fat source in fish diets. The replacement of fish meal with BSFL meal can be done up to 100% in the diets of Jian carp without unfavorable effects on growth while decreasing n-3 highly unsaturated fatty acid (HUFA) composition in the body of fish.12 Also, Lan et al.13 reported that using BSFL to replace trash fish in diets reduced harvesting size and the productivity of seabass.
Therefore, the study was designed to determine the effects of including fresh or dried BSFL in diets on the growth performance and chemical composition of snakehead fish cultured in small-scale farms.
Materials and Methods
Experimental animals and acclimation
The fingerlings of snakehead fish were purchased from a local hatchery, given a quarantine certificate, located in Thuan Loc ward, Hue City, Vietnam. The initial size of the fingerling of 5.15 ± 0.12 g in weight and 8.0 ± 0.17 cm in length. Fingerlings were acclimated to the experimental condition for two weeks.
Experimental net cages
Fifteen experimental net cages (capacity of 6 m3 per cage) were made of monofilament mesh (2a = 2 mm) and placed in a local farmer’s aquaculture pond in Huong Chu ward, Hue City, Vietnam. The water level in net cages was always maintained above 1.5 m, and the distance between the net cages was 30 - 50 cm.
Experimental feed
Commercial feed: Using commercial feed for freshwater fish produced by the Limited Liability Company CP, Vietnam. Code: CP9991 (Table 1).
Fresh black soldier fly larvae (Fresh BSFL): Biomass of BSFL was raised at a local farm located in Huong Chu ward, Hue City, Vietnam. After 10 – 12 days, fresh BSFL was harvested, removed from solid waste, and washed with clean tap water several times to remove dirt on the larvae’s bodies. Clean Fresh-BSFL was directly used for fish.
Dried black soldier fly larvae (Dried-BSFL): Fresh-BSFL were dried at 60˚C (using an Oven VN-100, Vietnam) for 48 hours, then cooled at room temperature and stored at 4 ˚C until feeding the fish (Table 1).
Experimental design
The experiment was conducted as a completely randomized design (CRD) in fifteen net cages (capacity of 6 m3) and was placed in an aquaculture pond at Huong Chu ward, Hue City, Vietnam. Including five (5) dietary treatments named: NT1 (100% commercial feed served as a control); NT2 (100% fresh BSFL); NT3 (100% dried BSFL); NT4 (50% fresh BSFL + 50% commercial feed); and NT5 (50% dried BSFL + 50% commercial feed). Each treatment was performed in triplicate. The amount of feed in the diets was determined by the ratio of fresh BSFL/dry BSFL = 3/1. Fingerlings of snakehead fish were stocked at a density of 20 fish.m-3. The experiment was carried out for 60 days.
Feeding and management
Snakehead fish were fed four times per day (7h, 10h30, 14h30, and 18h), with the amount of feed at 8% body weight. Fresh and dried BSFL were added following a regime at 10h30 and 18h daily. All leftover feed (commercial feed and BSFL) was removed from the net cages after 1h30 of feeding, put in an oven (under 60oC for four hours), and then the amount of uneaten feed was determined. Maintaining the water level in the pond was always above 1.5 m. Every two weeks, lime bags (5 kg.bag-1) were hung in the net cages to prevent diseases in fish.
Measurements
Water quality parameters such as temperature (˚C), pH, dissolved oxygen (DO), and N-NH3 were measured periodically (Table 2).
Survival rate (SR, %)
SR (%)=NtNo ×100
Where: No is a number of fish at the initial point of the experiment; Nt is a number of fish at the final point of the experiment.
Growth performance
Ten (10) fish in each net cage were randomly collected and anesthetized with Aqui-S® (concentration of 10 mL.m-3) biweekly to determine the growth performance. Then live weights of the fish were measured and recorded at the beginning and every fifteen days until 60 days. At the end of the experiment, ten (10) fish in each treatment were collected and stored at -20 ˚C for further analysis of the chemical composition of the fish.
Daily weight gain (DWG, g.day-1):
DWG (g.day−1)=Wi−Wot
Where: Wo and Wi are the live weight of the fish on the initial day and day i, and t is the period of the experiment.
Daily Length gain (DLG, cm.day-1):
DLG (cm.day−1)=Li−Lot
Where: Lo and Li are the body length of the fish on the initial day and day i, and t is the period of the experiment.
Feed intake (FI, g.fish-1) was determined by a ratio between the amount of feed intake (FI - in dry matter) and the number of surviving fish (SF).
FI (g.fish−1) =FISF
Feed conversion ratio (FCR) was determined by a ratio between the amount of feed intake (FI - in dry matter) and weight gain (WG).
FCR =FIWG
Protein efficiency ratio (PER) was determined by a ratio between the weight gain of fish (WG) and protein intake (PI).
PER =WGPI
Lipid efficiency ratio (LER) was determined by a ratio between the weight gain of fish (WG) and lipid intake (LI).
LER =WGLI
Condition factor (K) of fish:
K=100 x WL3
Where: W and L are the body weight and length of the fish. The K value is used to determine the health condition of the fish.
Yield (kg.m-2) was calculated by the total live weight of fish collected at the terminated experiment per square meter of water surface.
Yield =Total live weight of fishm2
Chemical composition analysis
At the end of the experiment, 10 fish in each cage were sampled for meat sampling. The fillet meat of 10 fish was taken out and mixed carefully before sampling.
The parameters of dry matter (DM), crude protein (CP), ether extract (EE), crude fiber (CF), and total ash of BSFL and the snakehead fish were analyzed according to the following methods: Dry matter content according to AOAC standard 930.15, 1990; Total ash content analyzed according to AOAC 942, 1990; The crude fiber content was analyzed according to the standard TCVN 4329 - 2007; Ether extracts content was analyzed according to AOAC 930.15, 1990; Total nitrogen content was analyzed according to AOAC standard 930.15.1990 and crude protein = 6.25 x N. Samples were analyzed at the Laboratory of Analysis of Feed and Livestock Products of the National Institute of Livestock Production, Ha Noi, Vietnam.
Statistical analysis
Data were presented in the form of the mean (M) and standard error of the mean (SEM). The data were statistically processed by analysis of variance (ANOVA) by the General Linear Model in Minitab v.16.2 (2010). The difference between the mean values was determined by the Tukey method at a confidence level of 95%.
Results
Water quality
Water quality parameters such as temperature (23.5 - 33.0˚C), pH (6.8 – 7.78), DO (4.01 – 4.80 mg.L-1), and NH3 (0.01 – 0.09 mg.L-1) was similar among treatments (net cages were placed in the same pond) (Table 3).
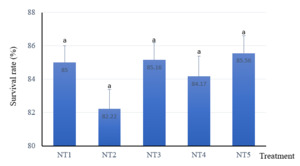
Survival rate
In this study, snakehead fish survival varied from 82.22 % to 85.56 % (Fig. 1). There was no significant difference among treatments (p>0.05). This proves that the snakehead fish could use and digest the experimental diets either with the inclusion of 100% fresh or dried BSFL.
Growth performance, feed utilization, yield, and condition factor (K)
After 15 days of culture, the growth in weight of snakehead fish began to differ between treatments (Fig. 2). Live weight fluctuated from 11.96 g.fish-1 to 15.03 g.fish-1. While, fish grown in NT4 (15.03 g.fish-1) had the best growth performance and were statistically significant differences as compared to those in NT2 (11.96 g.fish-1), NT3 (13.0 g.fish-1), and NT5 (13.16 g.fish-1) (p<0.05). However, there was no significant difference as compared to those in NT1 (14.70 g.fish-1). At this stage, fish adapted to the cultural environment thus the growth performance was still slow. However, after 30 days, the snakehead fish began to grow fast in live weight and showed a significant difference between the feeding treatments. The highest live weight of snakehead was found in NT4 (38.42 g.fish-1) and had a statistically significant difference as compared to those in NT2 (25.98 g.fish-1), NT3 (28.99 g.fish-1), and NT5 (30.70 g.fish-1). However, there was no difference as compared to the fish in NT1 (35.03 g.fish-1). While, the live weight of the fish in NT2 had the lowest growth performance and was a statistically significant difference as compared to those in the other treatments, except for the fish in NT3 (p>0.05).
At the end of the feeding trial, the final weight (FW), daily growth rate (DGR), and yield of snakehead were the highest in NT4 (103.3 g, 1.64 g.day-1, and 1.73 kg.m-2, respectively) (Table 4). These growth parameters were affected by the experimental diets. There were significant differences in growth performance between NT4 and NT2 (70.9 g, 1.09 g.day-1, and 1.16 kg.m-2, respectively), NT3 (85.1 g, 1.33 g.day-1, and 1.45 kg.m-2, respectively), NT5 (89.2 g, 1.40 g.day-1, and 1.50 kg.m-2, respectively) treatments (p<0.05), but no significant difference was found between NT4 and NT1 (101.10 g, 1.60 g.day-1, and 1.72 kg.m-2, respectively) (p>0.05). Predictably, the final length (FL) of snakehead fish was the highest in NT1 (22.83 cm) and was significantly different as compared to NT2 (20.07 cm), NT3 (21.86 cm) and NT5 (22.13 cm) (p<0.05). However, no significant difference was found between NT1 and NT4 (22.56 cm). Likewise, the daily length rate (DLR) of snakehead fish was also the highest in NT1 (0.25 cm.day-1) and was significantly different as compared to NT2 (0.20 cm.day-1) and NT3 (0.23 cm.day-1). However, no significant difference was found as compared to NT4 (0.24 cm.day-1) and NT5 (0.24 cm.day-1) (p>0.05).
The experimental diets significantly influenced feed utilization parameters (Table 4). Naturally, FI differed among the experimental treatments. Fish fed with the commercial diet (NT1) took the highest amount of feed (141.43 g.fish-1) and was significantly different as compared to NT2 (97.68 g.fish-1), NT3 (110.48 g.fish-1) and NT5 (117.24 g.fish-1). However, there was no significant difference between NT1 and NT4 (133.25 g.fish-1) (p>0.05). Interestingly, fish fed the diets with either the inclusion of 50% fresh or dried BSFL significantly improved FCR (NT4, 1.34) and (NT5, 1.40), respectively, and significantly differed as compared to NT1 (1.47) and NT2 (1.49). Contradictorily, fish fed the diets with the inclusion of fresh and dried BSFL statically reduced PER and LER by 15% (NT4), 22% (NT5) and 64% (NT4), 69% (NT5), respectively as compared to those fed with the commercial diet. However, it significantly improved PER and LER by 38% (NT4), 22% (NT5) and, 98% (NT4), 60% (NT5), respectively, compared to those fed with either the fresh or dried BSFL diets.
In the same trend, condition factor (K) was significantly affected by the experimental diets. The highest value of K was found in NT4 (0.91) and significantly different as compared to NT3 (0.83) and NT5 (0.82). However, no significant difference was found among NT4, NT2 (0.88), and NT1 (0.85) (p>0.05).
Chemical composition of snakehead fish
Proximate composition of fillet meat of snakehead at the end of the experiment, including the contents of dry matter (24.85 - 25.65 %), crude protein (15.35 - 15.75 %), crude lipid (5.31 - 5.57 %), and ash (3.22 - 3.51 %) was not affected by the inclusion of fresh and dried BSFL in the diets’ snakehead fish (p>0.05) (Table 5). The results indicated that the inclusion of fresh and dried BSFL up to 50% in the diet of the snakehead fish has no negative influence on the proximate composition of the fish.
Discussion
In this study, water parameter values seemed to be stable throughout the experimental period and were within the optimum ranges for growing snakeheads, according to Kiem and Long.14 Thus, mortality probably resulted from the behavior of the fish. Snakehead fish are carnivorous species, preferring to hunt fresh animals. They also have the habit of eating each other.1 The survival rate in this study was in agreement with a previous study by Hoa and Dung2 that grew snakehead (Chana micropiltes) directly using fresh BSFL and obtained a survival rate of 84.0 %. However, these results of survival rate were higher than that of 75.33 – 77.33 % of snakehead fish (Channa striata) reported by Mehrajuddin et al.15 and Chandan et al.,16 who grew snakehead fish (Channa striata) by diets that contain different levels of protein, obtained the survival rate of 62.0 – 68.7 %. On the contrary, the obtained survival rates were lower than that of 92.5 - 94.6 %, as reported by Saputra et al.17 regarding snakehead (Channa striata) in the recirculation system.
Better growth parameters in NT4 probably resulted from a diet rich in essential fatty acids, including fresh BSFL. Lan et al.13 reported that BSFL fed by tofu by-products contained very high LA (omega-6) ranging from 27.57 - 29.7 % and ALA (omega-3) ranged from 1.89 - 2.04 % as total fat. BSFL meal contains 2.7 % omega-3 fatty acids and 22.31 % omega-6 fatty acids as total fat.10 Meantime, fishmeal just contained 1.1 - 1.3 % linoleic acid (LA) and alpha-linolenic acid (ALA) 0.3-0.9% as total fat (Cho and Kim, 2011). Also, it relates to the habit of snakeheads, who prefer catching fresh insects. This may induce fish to catch and digest feed. In the present study, the feed intake (FI) data in NT4 (based on dry matter) was similar to that in NT1. In reality, fish in NT4 were fed a 50% fresh BSFL inclusion diet. Thus, the value of FI in NT4 was nearly two times larger (the ratio of fresh BSFL/dry BSFL = 3/1) than that in NT1. Values of PER and LER also proved the inclusion of 50% fresh BSFL enhancing the digestive ability of snakehead fish by 38%, 34%, and 10%, and 98%, 85%, and 16%, respectively, as compared to those in NT2, NT3, and NT5. Moreover, the condition factor (K) is used to determine the health condition of the fish. In this study, feeding snakehead fish with a diet of 50% fresh BSFL (NT4) gave the highest K value (close to 1.0). Nash et al.18 reported that the K value of 1.0 was favorable for growing snakeheads. A higher K value in NT4 may indicate the availability of favorite food and low competition of the fish for food in the culture system.19 Lower K values in NT3 and NT5 were closely related to biological interaction involving intraspecific competition for food and space between the fish.20,21 This may result in lower growth performance in those treatments.
In this study, the results of growth parameters such as FW (70.9 - 103.3 g) and DGR (1.09 - 1.64 g.day-1) were much higher than those of 5.6 - 7.8 g and 0.13 - 0.14 g.day-1 of snakehead (Channa striata) grown in the circulation system reported by Saputra et al.17 and snakehead (Channa striata) culture in the earthen pond reported by Rahman et al.22 obtaining FW (4.70 - 5.06 g) and DGR (0.14 - 0.16 g.day-1). This may be due to the different sizes of snakehead at the stocking point, in this experiment the fish were bigger than that in their experiments. These results of growth parameters were consistent with the study reported by Hoa and Dung,2 who grew snakehead fish (Channa micropeltes) on fresh BSFL, obtained FW (70.7 - 102.2 g) and DGR (1.02 - 1.67 g.day-1). FCR in this study ranged from 1.34 - 1.49. These results were in agreement with a study reported by Boonkusol and Tongbai23 obtaining FCR from 1.28 - 1.39. However, these FCR results were much lower than that of 3.05 - 4.06 recorded by Hoa and Dung,2 2.05 - 2.59 reported by Rahman et al.,3 and 3.90 - 4.77 documented by Saputra et al.17 The results proved that the inclusion of fresh BSFL at a rate of 50 % in the diet of the snakehead fish has not only reduced feed cost but also improved FCR and enhanced the growth performance of the fish.
In this study, including fresh and dried BSFL did not affect the proximate composition of snakehead fish. The replacement of dietary fish meal with BSFL did not alter the body composition of Jian carp (Cyprinus carpio var. Jian), as Zhou et al.12 reported. The results of the body composition of snakehead fish similar to the findings also worked with snakehead fish (Channa sp.) by Sagada et al.,24 were lower than those reported by Hua et al.25 and Yousuf et al.26 On the contrary, the inclusion of BSFL in the diets of climbing perch (Anabas testudineus Bloch, 1792) increased the dry matter content of meat in BSFL prepupae diets (36.9 -37.2 %). Meantime, the dry matter in the fish meal diet was just 36.6 %.27
In conclusion, including fresh or dried BSFL in the diets of snakehead fish during 60 days of feeding did not affect the growth performance, condition factor (K), and chemical composition. However, it reduced PER and LER. The results of the present study indicate that including fresh BSFL at a rate of 50% in the diet of snakehead improved FCR and maintained good condition factor, enhancing the growth performance and health status of the fish.
This research could also promote the sustainable development of the snakehead culture industry by finding a candidate insect-based feed that is partially replaced by 50% commercial feed in the diets of the fish. It suggests that farmers should feed their snakehead with commercial feed plus fresh BSFL to maintain the fish’s good health and enhance their growth performance and production.
Acknowledgments
This research was funded by Thua Thien Hue Department of Science and Technology under Grant No. TTH.2021-KC.26.
Ethical approval - IACUC
This experiment was conducted at La Chu Research Farm in Hue City, Vietnam, from May to July 2023 and was approved by the Animal Ethics Committee of Hue University No. HUVN0026.
Authors’ Contribution
Conceptualization: Hoang N. Manh (Equal), Nguyen D.Q. Tram (Equal). Data curation: Hoang N. Manh (Equal), Tran T.T. Suong (Equal), Pham T.P. Lan (Equal). Formal Analysis: Hoang N. Manh (Lead). Methodology: Hoang N. Manh (Equal), Nguyen D.Q. Tram (Equal). Writing – original draft: Hoang N. Manh (Lead). Investigation: Tran T.T. Suong (Equal), Pham T.P. Lan (Equal). Supervision: Nguyen D.Q. Tram (Lead). Writing – review & editing: Nguyen D.Q. Tram (Lead).