Introduction
Skin ulceration syndrome is the most significant disease in sea cucumber Apostichopus japonicus aquaculture that occurs in the wintering and seedling stages of A. japonicus breeding. The disease is mainly caused by bacterial infection, often accompanied by secondary infections caused by molds and parasites. Skin ulceration syndrome peaks from November to April of the following year. During periods of high temperature, the disease is characterized by rapid onset, high morbidity, and high mortality. In the early stage of infection, A. japonicus exhibits head shaking, followed by swelling and inability to open or close the mouth. In the middle stage of infection, the body shrinks, stiffens, and changes to a dark, cloudy color; the tube feet become blunt and white; small ulcers appear on the body surface; and many expel their viscera.1 In the late stage of infection, the lesions increase in number and expand, the number of ulcers increases, the ulcer surface expands, deep bluish-white ulcers appear on the body wall, and infected sea cucumbers finally die, becoming deliquescent.
The outbreak of the disease is caused by substrate pollution. In the aquaculture of A. japonicus, feces, and residual bait accumulate at the bottom of the pond, and the concentration of ammonia, nitrogen, and nitrite in the water body increases, creating favorable conditions for the reproduction of pathogenic bacteria. As the concentration of dissolved oxygen in the water body decreases, particularly below 2 mg/L, the immunity of A. japonicus decreases, and the nervous and digestive systems become more susceptible to infection by pathogens.2 On the other hand, incomplete disinfection of aquaculture ponds before stocking creates conditions for microorganism growth and disease outbreaks. Some aquaculture farms use antibiotics and disinfectants indiscriminately during the breeding process, which disrupts the micro-ecological balance of the aquaculture water body and inside the A. japonicus gut, thus decreasing the immunity of A. japonicus.3 In addition, excessive aquaculture rearing density and crowding effects can lead to slow growth, decreased viability, and reduced stress response in A. japonicus. Moreover, higher amounts of bait result in increased residual bait and feces in the water body and increase water pollution.4 Deterioration of aquaculture water quality affects growth rate, reduces resistance, and accelerates the spread of skin ulceration syndrome in A. japonicus. In addition, many breeding operations have not systematically selected and improved parental A. japonicus stocks and have been breeding them for many generations. Thus, the problem of broodstock degradation has become increasingly prominent, and the resulting seedlings have significantly reduced resistance.5 Most seedling purchasers do not have the technique or experience for testing the health of seedlings, which prevents them from selecting robust seedlings, thereby increasing the risk of disease transmission. Probiotics promote host health by enhancing the immune system, improving the biological environment, promoting digestion, and maintaining the balance of microorganisms in the body.6 Currently, probiotics are widely used in disease prevention and control in aquaculture, but there have been few reports on disease prevention and control in A. japonicus aquaculture.
In this paper, the effects of supplementing the feed of A. japonicus with the probiotics Lactobacillus, Bacillus, and a mixture of the two on the growth and immune indicators of A. japonicus, as well as on its resistance to skin ulceration syndrome were analyzed and compared, to discover an effective defense measure against the skin ulceration syndrome of A. japonicus.
Materials and Methods
Experimental animals
Yellow River Delta Marine Science and Technology Co., Ltd obtained A. japonicus sea cucumbers weighing about 40 g and 6 cm-7 cm in length. The sea cucumbers were placed in 10 PVC tanks (3m × 6m × 1m) for 2 weeks and offered daily basic feed (primarily seaweed powder). The appearance of regular feeding indicated the completion of domestication.
Experimental reagents
Protein testing kit, lipase, amylase, and trypsin kit (Nanjing, China). Probiotics included Lactobacillus and Bacillus preparations (Shandong Zhongke Jiayi, China) Pathogenic bacteria included Vibrio splendidus and Pseudoalteromonas (Corporate Laboratory Preservation).
Study design
Animals were divided into 4 experimental groups: a control group offered basic feed, experimental group 1 with 1% (w/v) of Lactobacillus added to the feed, experimental group 2 with 1% of Bacillus added to the feed, and experimental group 3 with 0.5% of Lactobacillus and 0.5% of Bacillus added to the feed. Each group included three replicates and was stocked in parallel with 100 sea cucumbers. The water was changed in the morning. About 1/3 of the total amount of water was changed each time, and the water was continuously aerated. Baiting occurs once daily at 6 PM for 60 days. Water quality indicators such as ammonia nitrogen, nitrite, dissolved oxygen, and pH were monitored daily during this period.
Specimen collection
Sea cucumber intestines were collected 24 h after feeding was stopped. Three sea cucumbers from each group were collected and then dissected with sterilized surgical knives. The intestines were harvested, separated, and labeled in sterile 2 mL EP tubes and stored at -80 °C until use.
Determination of growth performance
After feeding with feed containing probiotics for 60 d, the sea cucumbers were weighed, and the specific growth rate, feed conversion efficiency, feed intake, survival rate, and relative weight gain rate were calculated.
Calculations
Specific growth rate (SGR) = 100(LnW2 - LnW1) / t
Feed conversion efficiency (FCE) = 100(W2 - W1) / F
Survival rate = (final number of surviving individuals / initial number of individuals) × 100%
Weight gain rate (WGR) = 100(W2 - W1) / W1
Feed intake (FI) = F / t / final number of surviving individuals
Where W1 and W2 are the weights (g) of the sea cucumbers at the beginning and end of the experiment, respectively; t is the experimental length (day); and F is the amount of feed taken in by the sea cucumbers over the experimental period (g).
Determination of A. japonicus intestinal digestive enzyme activity
Sea cucumber intestinal homogenate experimental samples were assayed using commercial kits to determine their lipase, amylase, and trypsin activities per the manufacturers’ instructions.
Determination of A. japonicus coelomocyte phagocytic activity
Three sea cucumbers per group were collected every 20 d after the start of the experiment and injected dorsally with 0.1 mL of dried yeast cell suspension prepared with sterile seawater (0.1 mg/mL). After 4 h, coelomic fluid slides were collected using a syringe, and coelomocytes were counted. The phagocytosis rate was calculated as follows.
Phagocytic rate = number of coelomocytes with phagocytosis / total number of phagocytic cells × 100%.
Infection resistance experiments
The sea cucumbers were infected with V. splendidus and Pseudoalteromonas. After giving feed supplemented with microecological preparations for 2 months, each group of sea cucumbers was then artificially infected with V. splendidus and Pseudoalteromonas. 0.1 mL of bacterial suspensions (concentration: 3.7 × 108 cfu/mL) were injected dorsally into sea cucumbers, with 10 sea cucumbers injected per group; an equal amount of sterile saline was injected as a blank control. Next, the sea cucumbers were placed in a clean PVC tank and observed for 7 d; the rearing conditions were the same as before injection. The morbidity and mortality of the sea cucumbers were observed and recorded several times per day. Dead sea cucumbers were dissected, bacteria were isolated, and the cause of death was confirmed. The following calculations were performed:
Relative protection rate = (1 – inoculated group mortality rate – control group mortality rate) × 100%
Data processing
One-way ANOVA of the experimental data was performed using SPSS 22.0 software. Duncan’s multiple comparisons test was used in cases where statistically significant differences among the treatment groups were present (p < 0.05). Experimental data are expressed as mean ± standard deviation, and statistically significant differences are indicated by different lowercase letters.
Results
Effect of probiotics on the growth of A. japonicus
The sea cucumbers’ SGR, FCE, survival rate, and WGR in the probiotic supplementation groups were significantly greater than in the control group. In addition, these values were higher in the mixed probiotic supplementation group than in the single probiotic supplementation groups. The WGR was 120.08 ± 12.07% in the control group, 152.11 ± 10.06% in the Lactobacillus group, 148.03 ± 9.88% in the Bacillus group, and 210.11 ± 19.41% in the mixed probiotic group (Table 1).
Digestive enzyme activity in the A. japonicus intestine
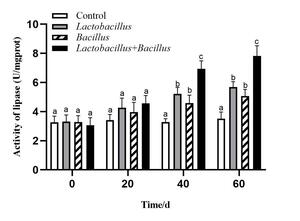
The activities of intestinal digestive enzymes of sea cucumbers in each treatment group are shown in Figures 1-3. The intestinal protease, amylase, and lipase activities of sea cucumbers in the Lactobacillus and Bacillus groups were significantly higher than in the control group at 40 d and 60 d. Digestive enzyme activities were higher in the Lactobacillus group than in the Bacillus group. Lipase and amylase activities in the mixed probiotic supplementation group were higher than those of the single-probiotic supplementation and control groups at 40 d and 60 d (p < 0.05).
Effect of microecological preparations on A. japonicus coelomocyte phagocytosis
Figure 4 shows that probiotics can significantly increase the phagocytic rate of A. japonicus coelomocytes. The results showed that feed supplementation with Lactobacillus and Bacillus significantly affected the phagocytic rate of coelomocytes (p < 0.05), with the control group having a consistently lower phagocytic rate. The mixed probiotic supplementation group was higher than the single probiotic supplementation groups and the control group (p < 0.05).
Effect of microecological preparations on immune protection rate in A. japonicus
The results showed that the control group exhibited poor attachment and was gathered at the bottom of the tank on day 1 after infection. In contrast, the three groups with probiotic supplementation still had good mobility, and almost all were attached to the sidewalls of the tanks. The control group showed mortality on day 1 after infection, whereas the mortality rates of the three groups with probiotic supplementation were decreased compared to the control group. The results of the effect of probiotics on the relative protection rate of the sea cucumbers are shown in Figure 5. ANOVA showed that the effect of probiotics on the immune protection rate was significant p < 0.05). The magnitude of the immune protection rate of each group was in the order: mixed probiotic supplementation group > Lactobacillus group > Bacillus group, at 85%, 60%, and 40%, respectively.
Discussion
Feed supplementation with probiotics promoted the growth of A. japonicus, improving their growth and weight gain rate. The weight gain rates of the control, Lactobacillus, Bacillus, and mixed probiotic supplementation groups were 120.08 ± 12.07%, 152.11 ± 10.06%, 148.03 ± 9.88%, and 210.11 ± 19.41% respectively. The experiment showed that the effect of Lactobacillus was better than Bacillus and the mixed probiotic supplementation was better than that of single probiotic supplementation.
Feed supplementation with probiotics improves the activity of digestive enzymes in the intestines of aquatic animals and promotes digestion and nutrient absorption. The activity level among digestive enzymes and amylase was marginally higher in the Bacillus compared to the Lactobacillus, and the combination of probiotics proved more potent than a probiotic used singularly. Additionally, the activity of both enzymes increased slowly over time.
Feed supplementation with probiotics significantly improved the phagocytic rate of A. japonicus coelomocytes. The phagocytic rate of Lactobacillus and Bacillus subtilis was comparable, with Lactobacillus exhibiting slightly higher levels than Bacillus subtilis. The mixed probiotics were significantly higher than single probiotics, and this phagocytic rate gradually increased.
Feed supplementation with probiotics improved immunity and enhanced resistance against Vibrio splendidus and Pseudoalteromonas infection. In addition, probiotic supplementation also has good bacteriostatic and bactericidal effects on pathogenic bacteria. According to the experimental results, Bacillus exhibits superior antimicrobial ability to Lactobacillus, and the mixed strain displays the most effective antimicrobial properties.
The present study demonstrated that probiotics regulate the microecological environment in A. japonicus’s body and its aquatic biological environment, improving the activity of digestive enzymes in the intestinal tract and dramatically improving nutrient absorption. Furthermore, probiotics also significantly improved antimicrobial activity in A. japonicus tissues and phagocytosis by coelomocytes, significantly enhancing resistance to skin ulceration syndrome pathogens. Lactobacillus promotes nutrient absorption, enhances phagocytosis, and boosts host immunity, indicating that Lactobacillus is a suitable probiotic strain for feed supplementation.7 Bacillus’s physiological activity or metabolites can promote growth and development, stimulate the immune system, and ameliorate various stress-induced disorders. Single probiotics and their combinations play comparable roles in enhancing immunity and promoting growth, but probiotic combinations demonstrate greater efficacy than single probiotics.8
According to the current farming mode and disease characteristics of sea cucumber, disease prevention should be prioritized. Based on the trend of comprehensive experimental indicators, it can be observed that in future farming processes, providing fresh and clean feed supplemented with a mixture of probiotics can effectively enhance sea cucumbers’ immune system and disease resistance.
In addition to using probiotics to alleviate skin ulcer syndrome in sea cucumbers, it is crucial to implement comprehensive prevention and control measures. These measures can be implemented mainly from the following perspectives. On the one hand, selecting healthy seedlings for pre-release health inspections is vital to ensure they are free of pathogens when entering the pond. During visual inspections, it is advisable to select sea cucumber seedlings with intact body surfaces, naturally elongated bodies, strong attachment, active feeding behavior, and dry feces in stripes.9 If conditions permit, further confirmation should be performed for their health status through methods such as microscopic observation. On the other hand, developing completely formulated diets to improve feeding efficiency is desirable. This includes researching sea cucumbers’ digestive physiology and nutritional requirements, developing specially formulated feeds for sea cucumbers, and improving feed processing technology to produce environmentally friendly feeds with high stability, palatability, absorbability, and low feed conversion ratio (FCR). This should also include ensuring the freshness and cleanliness of feeds while increasing the feeding rate of sea cucumbers to improve the benefits of cultivation.10 Additionally, it is crucial to enhance farming models by implementing specific measures. This involves carefully designing culture ponds and considering the appropriate stocking densities for seedlings. To promote the well-being of sea cucumbers during the winter season, it is recommended to adopt a “winter disease fall treatment” strategy. This entails regularly applying substances such as potassium hydrogen sulfate or other bottom substrate enhancers. This approach aims to facilitate the oxidation of organic matter present in pond sediment waste. Doing so creates a conducive environment, allowing sea cucumbers to overwinter safely.11 Furthermore, it is essential to actively explore innovative farming models, such as cage culture or three-dimensional diversified aquaculture, that can effectively facilitate the rapid growth of sea cucumbers. Additionally, there is a need to enhance daily management practices, including the regular removal of sediment waste from the bottom of the aquaculture system. It is also advisable to consistently add water quality improvers to water sources as part of routine maintenance. This helps reduce the levels of toxic substances and hydrogen sulfide in the aquaculture water, contributing to a healthier and more sustainable sea cucumber farming environment.
To promote the sustainable development of the sea cucumber industry, it is recommended to strengthen research in the following areas. Improving breeding techniques and selecting high-quality varieties with strong resistance can further improve the production rate of superior breeds and enhance technical aquaculture.12 Establishing early warning systems for disease diagnosis by developing rapid detection technologies such as high-throughput gene detection chips and colloidal gold test strips. Develop biological products, including bacteriophages, egg yolk antibodies, immune enhancers, and plant-based tissue repair agents as antibiotics. This will establish a targeted and complementary combination of technology for disease control and treatment.13 Improving water purification technologies and water environment regulation by developing composite microbial preparations with multiple functions in combination with immobilization techniques for microbial strains.
Acknowledgments
This work was supported by the Shandong Modern Agricultural Industrial Technology System (grant number: SDAIT-22-03).
Authors’ Contribution per CRediT
Data curation: Cai-Yun Li (Equal), Tao Xu (Equal). Methodology: Cai-Yun Li (Equal), Ming Hu (Equal). Formal Analysis: Xiao-Ai Li (Lead). Investigation: Xiao-Ai Li (Lead). Writing – original draft: Xiao-Ai Li (Equal), Lin-Tao Tan (Equal). Writing – review & editing: Ning Zhang (Equal), Tao Xu (Equal). Resources: Yang Li (Lead). Funding acquisition: Ming Hu (Lead). Supervision: Ming Hu (Equal), Tao Xu (Equal). Conceptualization: Tao Xu (Lead).
Ethical Conduct Approval – IACUC
In this study, the animal experiments were permitted in advance by the Instructional Animal Care and Use Committee of Jinan Vocational College. All possible efforts were used to reduce the suffering of the experimental animals.