Introduction
Blastocystis sp. is a widely distributed protozoan that lives in the intestines of humans and animals. The host can be infected by eating contaminated food or water, causing gastrointestinal symptoms such as abdominal pain and diarrhea. Still, the pathogenicity of the parasite has always been controversial. Some speculated that Blastocystis sp. should be an important part of the gut microbiota and play a role in maintaining host immune homeostasis.1
Blastocystis sp. has a wide range of hosts, including primates, carnivora, artiodactyla, perissodactyla, proboscidea, diprotodontia, rodentia, aves, amphibians (frogs) and reptiles (snakes).2–4 Only a few studies have shown invertebrates could be the parasite vector, including earthworms, cockroaches, and houseflies.5–7 Puzzlingly, more than 33 subtypes of the parasite, identified according to the genetic sequence of SSU-rDNA, were detected in different groups of animals. Thus, it is indicated that Blastocystis sp. has rich genetic diversity and cryptic host specificity.8,9 For fish, little attention has been paid to Blastocystis sp. in aquatic animals, and available data is limited until now. There have been no reports of fish infecting Blastocystis sp. in China. In the Atlantic northeast and on the coasts of northern France, a study reported the distribution of Blastocystis sp. in edible marine fish and marine mammals for the first time and the zoonotic subtypes of the parasite in marine fish.10 To our knowledge, Blastocystis sp. has only been isolated from the intestines of freshwater fish in 1997, but the subtype has not been identified.11
Most animals out of water quality are not alive, so many researchers are doubtful whether the contaminated water spreads Blastocystis sp. To our happiness, after determining Blastocystis sp. in water, it has recently been listed as an important indicator of drinking water monitoring by the WHO.12 The parasite has been detected in tap, river, and seawater in Asia in the past decade and identified ST1, ST2, ST3, ST4, ST5, ST6, ST8, and ST10, among which ST1 and ST3 are most common in water.13 In Europe, similar surveys have shown that Blastocystis sp. was free-live in natural water, and the detection rate was up to 13.9% within one year. Similarly, the most popular subtypes were ST1 and ST3.14 However, further studies about the relationship between the parasite and fish, water as a medium, are needed. Considering the correlation between humans, animals, and environments, it is also necessary to carry on the epidemiological studies on Blastocystis sp. with respect to ecology.
In recent years, fish has been an important source of animal protein for nearly 7 billion people worldwide, and its demand is increasing yearly. The freshwater aquaculture industry has developed greatly, and cold-water fish culture is China’s most important aquaculture model. As experience accumulates, people realize the magnitude of the water quality. Microorganisms and plankton are important biological indicators for evaluating freshwater aquaculture environments.15,16 The purpose of this study was to survey the distribution of Blastocystis sp. in fish and water environments to assess the impact of the parasite on the healthy breeding of cold-water fish. The results will provide data support for ecological prevention and control of cold-water fish diseases and lay a foundation for the formulation of sustainable aquaculture development strategies.
Materials and Methods
Sample collection
From August 2020 to June 2022, a total of 27 cold-water fish intestines, from 12 Acipenser sinensis, 5 Oncorhynchus mykiss, and 10 Gymnocypris przewalskii, were collected from three cold-water fish farms in Shijiazhuang, Hebei province, China, and cleaned the intestinal surface with ultrapure water to ensure the accuracy of the experiment. The intestinal contents were squeezed into a sterilized EP tube. Water samples were divided into source water (before filling the ponds and not in contact with fish), aquaculture water (in the fish ponds), tail water (discharged from the fish ponds and flowed settling ponds) and purified water (filtered tail water) according to the source. 334 water samples, 50mL each, were extracted and filtered through disposable filters.
DNA Extraction
DNA extraction of fish intestinal contents and water environment samples was carried out by Stool Genomic DNA Extraction Kit and Animal Tissues/Cells Genomic DNA Extraction Kit (Solarbio Science & Technology Co., Ltd., Beijing, China), respectively. All operations followed the instructions strictly.
Amplification of the SSU rDNA of Blastocystis sp.
The SSU rRNA gene of Blastocystis sp. was amplified by PCR using the parasite-specific primers RD5-R: 5′-ATC TGG TTG ATC CTG CCA G-3′ and BhRDr-F: 5′-GAG CTT TTT AAC TGC AAC AAC G-3′.17 The 50 μL PCR reaction comprised 25 μL 2×EasyTaq PCR SuperMix (TransGen Biotech Co., Ltd., Beijing, China), 2 μL (10 μM) of each primer, 1μL DNA, and 20 μL ddH2O. The following amplification conditions were used: 94℃ for 2 min, followed by 30 cycles at 94℃-30 s, 58℃-30 s, and 72℃-45 s, with a final extension step at 72℃ for 5 min. To ensure experimental accuracy, both negative and positive controls were implemented. Blastocystis 18S rRNA gene, cloned on pEASY-Blunt vector (Transgen, Beijing, China), was used to by the positive control. Subsequently, 1.5% agarose gel was prepared to detect amplified products. The commercial supplier, Sangon Biotech (Shanghai) Co., Ltd, sequenced the anticipated products.
Sequence alignment and phylogenetic analyses
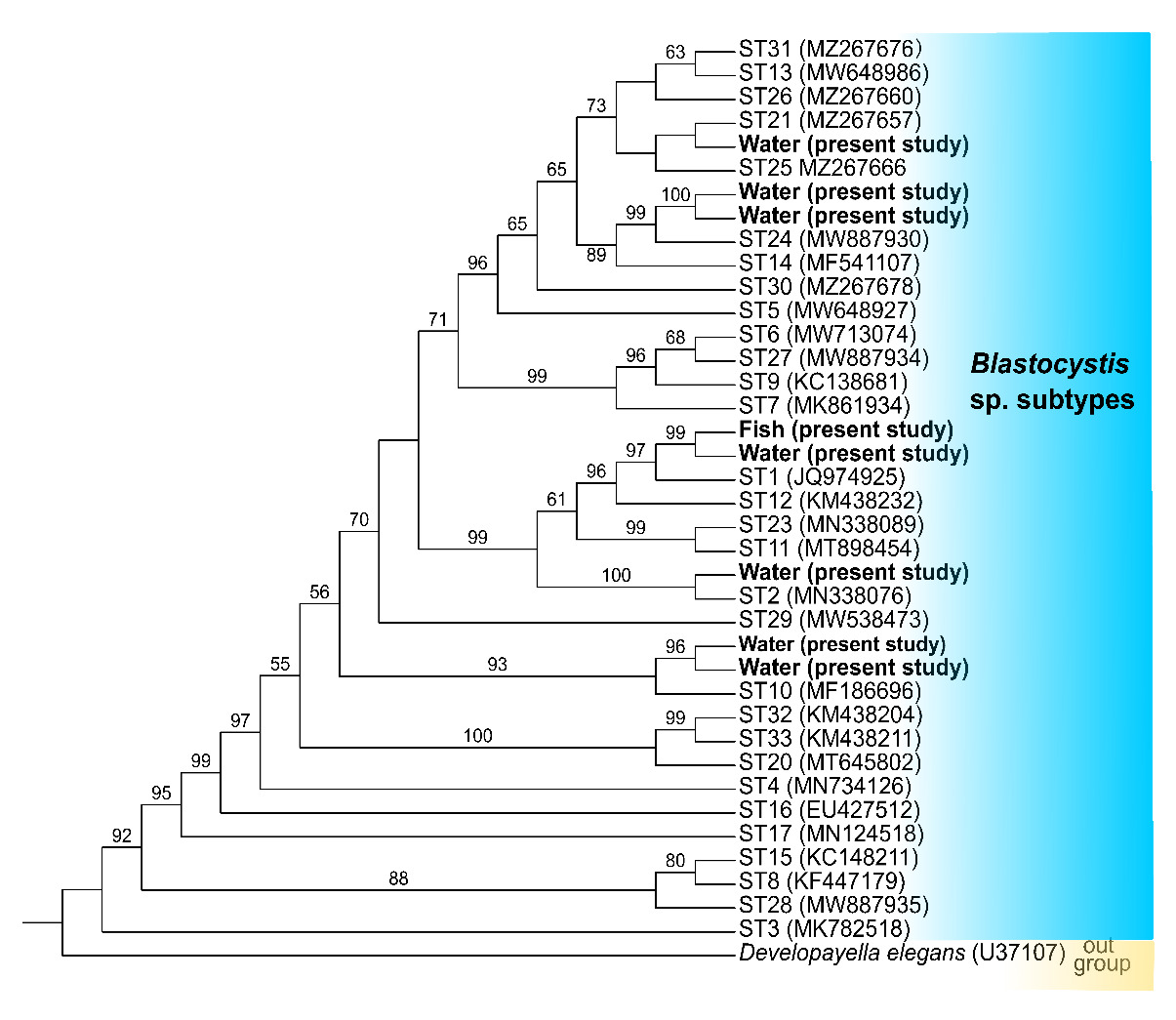
All positive nucleotide sequences of Blastocystis sp. were subjected to correction and compared with reference representing each ST in GenBank using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The above sequences and 33 reference sequences were aligned using MAFFT and then trimmed at both ends with BioEdit. Developayella elegans was selected as an outgroup. IQ-TREE (version 1.6.8) was used to construct the phylogenetic tree using the maximum likelihood method and tested with 1000 standard bootstrap replicates. iTOL v6.1.1 was employed to visualize the phylogeny and architecture with files generated by PhyloSuite. The resulting phylogenetic trees were further enhanced by visualizing the structure through iTOL, utilizing files generated by PhyloSuite.
Statistical analysis
We examined the infection rates of Blastocystis sp. in water samples, recording the data according to seasons and source. Statistical analysis was performed with SPSS (https://spssau.com/en/index.html). All data are expressed as mean ± standard deviation (SD). Differences were considered statistically significant when P <0.05.
Results
Occurrence of Blastocystis sp.
Of 27 cold-water fish intestines, three Acipenser sinensis were positive for Blastocystis sp. with a stripe size of about 600bp, consistent with the expected target size (Figure 1A) and the infection rate of 11.11%. Likely, 21 of 334 water environment samples were positive (Figure 1B), with a positive rate of 6.29%. All positive samples were identified as SSU rRNA gene of Blastocystis sp. by sequencing and blasting.
Subtypes and Phylogenetic analysis
Sequence analysis was performed for 24 PCR products to identify the subtypes of Blastocystis sp., and BLAST analysis confirmed the presence of five subtypes (STs), including ST1 in intestines and ST1, ST2, ST10, ST21, and ST24 in water. The comparability of the three sequences from fishes each other is 100%. Phylogenetic analysis showed the same results as BLAST (Figure 2).
Seasonal and distribution differences of Blastocystis sp.
The detected rates of Blastocystis sp. in the water environment in spring, summer, autumn, and winter were 7%, 6%, 6%, and 6%, respectively, and the statistical analysis results showed no significant differences among different seasons (P>0.05) (Table 1). For the four sources of water, it was found that Blastocystis sp. existed in the four water samples, and there were no significant differences in the parasite distribution from each other (Table 2).
Discussion
With the development of freshwater aquaculture, people are increasingly aware of the importance of the ecological environment of aquiculture and the sustainable development. Aquatic animals have the characteristics of short and simple digestive organs, poor digestive enzyme activity, and short retention time of food in the intestinal tract. The gut of fish is the most important digestive organ, and here, the food is decomposed into nutrients absorbed and utilized by the body.18 In addition, the gut participates in the process of immune function and host defense and is considered to be a “natural immune organ” in fish.19–22 Thus, maintaining intestinal health is more and more important. In the present study, Blastocystis sp. ST1 was detected by sequence and phylogenetic analysis, which suggested that cold-water fish might be one of the hosts of Blastocystis sp. Many studies have shown that protozoa could reduce the digestion efficiency of fish feed and affect the immune system, becoming vulnerable to other pathogens.23 To our knowledge, this was the first time Blastocystis sp. was detected in cold-water fish. Surprisingly, ST1 was shown in the water environment, the same as that of fish. It is speculated that Blastocystis sp. entered the intestinal tract of fish with feeding and water flow. As described in the results, there were ST2, ST10, ST21, and ST24 besides ST1 in water environments but only ST1 in the fish, and the reason for that was probably a wide host of ST1, specificity of other STs, and few fish tissue samples. The idea that Blastocystis sp. could exist in different water has been accepted according to previous studies, such as in treated wastewater, seawater, and irrigated water, and there were different subtypes in different areas.24,25 In addition, the presence of Blastocystis sp. in source and aquaculture water in this study indicated the potential role of this type of water in the transmission of pathogenic microorganisms to downstream environments. Meanwhile, the results reminded us that disease control and health management in aquaculture needed more advanced countermeasures of water quality to prevent some protozoan.
In recent years, with the adjustment of agricultural structure and the promotion of agricultural industrialization, the large-scale and intensive aquaculture industry has developed rapidly and become an important part of the agricultural and rural economies in developing countries.26–28 The development of aquaculture has met a large number of people’s needs. Still, it has also become an important pollution source of water pollution, which poses a huge potential threat to human health.29 Sewage purification has attracted more and more attention in the social context of environmental protection and energy saving. Establishing a perfect purification system is the only way to realize the sustainable development of pond pollution-free aquaculture and inland fishery.30,31 In the present study, Blastocystis sp. was detected in the tail and purified water, and the detected rate in the two water bodies did not show a significant difference, indicating contaminated water might be a source of human and animal infection. It also suggested that our purification system had a weak ability to purify harmful protozoa in fish
Acknowledgments
This work was supported by the S&T Program of Hebei (H2021205002, 22326703D) and the S&T Program of Shijiazhuang, Hebei (225500395A).
Authors’ Contribution per CRediT
Data curation: Yuwei Wang (Equal), Huizhu Nan (Equal). Investigation: Yuwei Wang (Lead). Writing – original draft: Yuwei Wang (Equal), Huizhu Nan (Equal). Methodology: Chao Zhang (Equal), Mengjuan Cao (Equal). Software: Chao Zhang (Equal), Shi Yin (Equal). Visualization: Chao Zhang (Lead). Project administration: Huizhu Nan (Equal), Lei Ma (Equal). Validation: Huizhu Nan (Lead). Supervision: Ruiyong An (Equal), Lei Ma (Equal). Writing – review & editing: Lei Ma (Lead).
Ethics statement
This study followed the guidelines of the Regulations for the Administration of Affairs Concerning Experimental Animals and was approved by the Biomedical Ethical Committee of Hebei Normal University, Hebei, China (ID: 2022LLSC024).

_detection_in_fish_intestines__(b)_detection_in.png)

_detection_in_fish_intestines__(b)_detection_in.png)